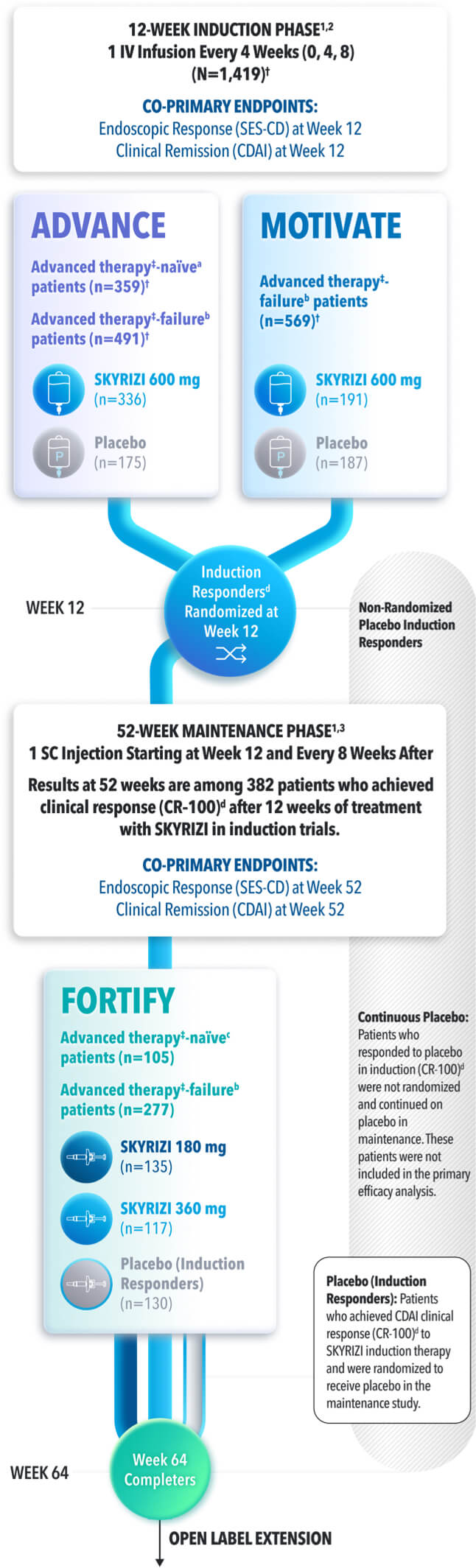
The IL-23 inhibitor from AbbVie indicated for the treatment of moderately to severely active Crohn's disease (CD) in adults.1
RESOURCES
FOR YOUR PATIENTS
AND YOUR PRACTICE
RESOURCES TO HELP GET YOUR PATIENTS TIMELY ACCESS TO SKYRIZI
BILLING AND CODING
Clear guidance on billing and coding for SKYRIZI, including NDC codes.

ACCESS AND REIMBURSEMENT
Forms and instructions to help patients with access and coverage.
Specialty Pharmacies and Distributors
Contact details for specialty pharmacies, wholesalers and specialty distributors.
This information is for informational purposes only and is not intended to provide reimbursement or legal advice. The information presented here does not guarantee payment or coverage.
 SKYRIZI COMPLETE ENROLLMENT
SKYRIZI COMPLETE ENROLLMENT
SKYRIZI COMPLETE ENROLLMENT AND PRESCRIPTION FORM
- Download and fill out the Skyrizi Complete Enrollment and Prescription Form with your patient.
 BILLING AND CODING
BILLING AND CODING
GUIDE TO BILLING AND CODING
Your guide to relevant codes (including commercial and Medicare) as well as helpful tips for completing forms.
 ACCESS AND REIMBURSEMENT FORMS
ACCESS AND REIMBURSEMENT FORMS
Along with support from Skyrizi Complete, you can use the forms here to help patients with access and coverage for SKYRIZI.
HIPAA AUTHORIZATION
Allow patients to authorize the release of health information related to their treatment with SKYRIZI.
ASSIST PATIENTS WITH SKYRIZI IV & ADMINISTRATION COSTS
Utilize this form as a guide when submitting patient claims for reimbursement, for eligible, commercially insured patients using the Skyrizi Complete Savings Card.
For support in person or over the phone with patient-specific access and reimbursement education, including prior authorization and appeal support, connect with your Field Reimbursement Specialist.
PRIOR AUTHORIZATION (PA) BROCHURE AND CHECKLIST
Use these helpful resources to assist with your patient PA submission requirements for SKYRIZI.
HIPAA=Health Insurance Portability and Accountability Act; IV=intravenous.
 SPECIALTY PHARMACIES AND DISTRIBUTORS
SPECIALTY PHARMACIES AND DISTRIBUTORS
IV=intravenous.