The IL-23 inhibitor from AbbVie indicated for the treatment of moderately to severely active Crohn's disease (CD) in adults.1
WELL-STUDIED Safety Profile
UP TO ~9 YEARS OF CLINICAL TRIAL EXPERIENCE ACROSS 4 INDICATIONS1,2
Approved for 4 Indications: Starting With Plaque Psoriasis (Ps), Followed by Psoriatic Arthritis (PsA), Crohn’s Disease, and Ulcerative Colitis (UC)1,2

34
Clinical Trials
Across Indications1,2*†
26,880
Patient-Years of Clinical Trial Exposure2*‡

325,458
Patients Prescribed Worldwide Since 2019 Starting With Ps3§

41,467
Patients Prescribed
Worldwide in Crohn’s3§
*Safety data were evaluated for all patients receiving ≥1 dose of SKYRIZI from Phase 1-4 trials, including open-label extension and dose-ranging studies.
WELL-STUDIED SAFETY PROFILE
Week 12 Safety Data from
Phase 2 and 3 Induction Trials1,5
| POOLED INDUCTION TRIAL DATA | ||
|---|---|---|
| Adverse Events (AEs) |
Placebo IV N=432 |
SKYRIZI 600 mg IV* N=620 |
| Treatment-Emergent AEs | % | % |
| Any AE | 63.4 | 54.7 |
| Serious AE | 15.5 | 6.6 |
| AE Leading to Discontinuation of Study Drug† |
8.6 | 2.1 |
| Death | 0.5 | 0 |
| AEs of Special Interest |
% | % |
| Infections | ||
| Infections | 24.8 | 19.7 |
| Serious Infection | 3.7 | 1.0 |
| Opportunistic Infection (Excluding TB/Herpes Zoster) |
0.7 | 0 |
| Active TB | 0.2 | 0.2 |
| Malignancy | ||
| Malignant Tumors (Including NMSC) | 0 | 0 |
| Cardiovascular Events | ||
| Adjudicated MACEa | 0 | 0 |
| Other | ||
| Hypersensitivity (Serious Events Only) | 0.2 | 0.2 |
| Adjudicated Anaphylactic Reaction | 0 | 0 |
| Hepatic Events | 1.6 | 1.6 |
| Infusion-Related Reactions | 1.2 | 0.8 |
SKYRIZI IS CONTRAINDICATED IN PATIENTS WITH A HISTORY OF SERIOUS HYPERSENSITIVITY REACTION TO RISANKIZUMAB-RZAA OR ANY OF THE EXCIPIENTS.1
In the CD 12-week induction studies, the most common adverse reactions (>3% of patients and at a higher rate than placebo) include upper respiratory infections, headache and arthralgia.1
WELL-UNDERSTOOD SAFETY PROFILE AND TOLERABILITY† WITH 2.1% OF SKYRIZI PATIENTS DISCONTINUING THE TRIAL DUE TO ADVERSE EVENTS9
WELL-STUDIED SAFETY PROFILE
Week 52 Safety Data from
FORTIFY1,6,7,8
SKYRIZI IS CONTRAINDICATED IN PATIENTS WITH A HISTORY OF SERIOUS HYPERSENSITIVITY REACTION TO RISANKIZUMAB-RZAA OR ANY OF THE EXCIPIENTS.1
In the CD 52-week maintenance study, the most common adverse reactions (>3% of patients and at a higher rate than placebo) include arthralgia, abdominal pain, injection site reactions, anemia, pyrexia, back pain, arthropathy, and urinary tract infection.1
WELL-UNDERSTOOD SAFETY PROFILE AND TOLERABILITY* WITH 2% OF SKYRIZI 180 MG SC PATIENTS AND 3% OF SKYRIZI 360 MG SC PATIENTS DISCONTINUING THE TRIAL DUE TO ADVERSE EVENTS7
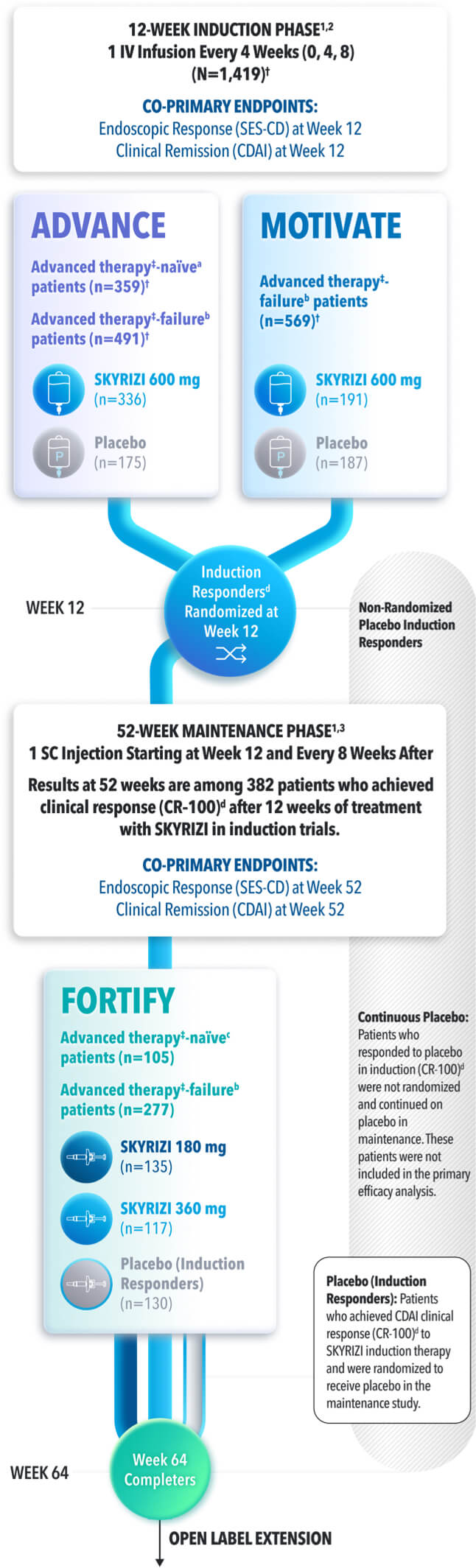
Continuous Placebo: Patients who responded to placebo in induction (CR-100)† were not randomized and continued on placebo in maintenance. These patients were not included in the primary efficacy analysis.
Placebo (Induction Responders): Patients who achieved CDAI clinical response (CR-100)† to SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.
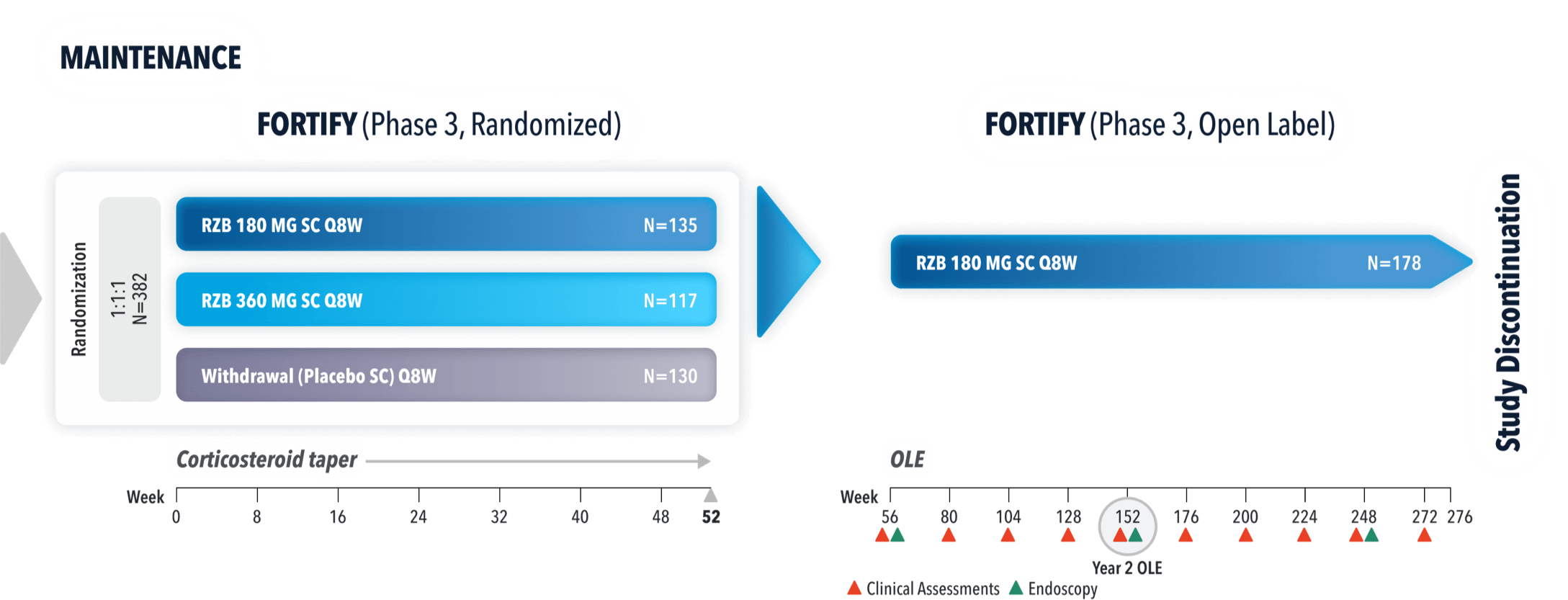
WELL-STUDIED SAFETY PROFILE
~3 Years of Safety Data from FORTIFY7,10,11
SKYRIZI IS CONTRAINDICATED IN PATIENTS WITH A HISTORY OF SERIOUS HYPERSENSITIVITY REACTION TO RISANKIZUMAB-RZAA OR ANY OF THE EXCIPIENTS.1
IN THE CD 52-WEEK MAINTENANCE STUDY, THE MOST COMMON ADVERSE REACTIONS (>3% OF PATIENTS AND AT A HIGHER RATE THAN PLACEBO) INCLUDE ARTHRALGIA, ABDOMINAL PAIN, INJECTION SITE REACTIONS, ANEMIA, PYREXIA, BACK PAIN, ARTHROPATHY, AND URINARY TRACT INFECTION.1
FORTIFY OLE STUDY DESIGN: AN ONGOING, TWO ARM, MULTICENTER, OPEN-LABEL EXTENSION OF PHASE 3 STUDIES EVALUATING THE LONG-TERM EFFICACY AND SAFETY OF SKYRIZI (180 MG OR 360 MG SC). PATIENTS WHO ACHIEVED CLINICAL RESPONSE IN ADVANCE OR MOTIVATE AT WEEK 12 AND COMPLETED THE 52 WEEK FORTIFY MAINTENANCE PERIOD WERE ELIGIBLE TO PARTICIPATE.7
LONG-TERM SAFETY DATA PRESENTED INCLUDES PATIENTS WITH VARYING LENGTHS OF TREATMENT EXPOSURE.
LONG-TERM SAFETY: INCLUDES DATA FOR PATIENTS WHO RECEIVED ≥1 DOSE OF SKYRIZI IN PHASE 1, 2, AND 3 CROHN’S STUDIES.
Placebo (Induction Responders): Patients who achieved CDAI clinical response (CR-100)† to SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.