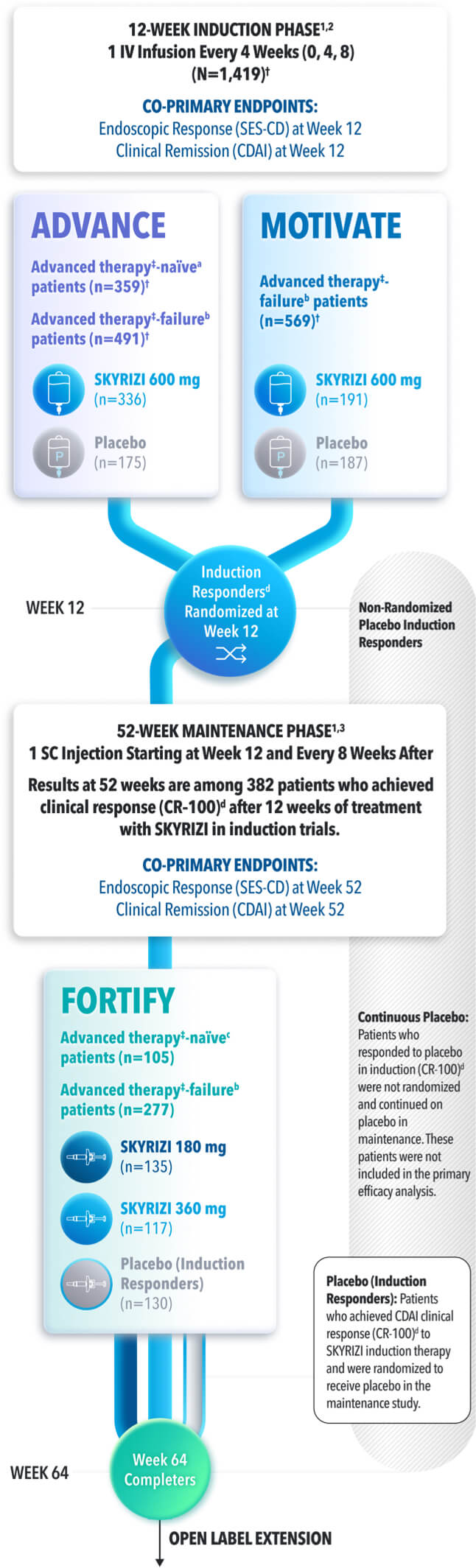
The IL-23 inhibitor from AbbVie indicated for the treatment of moderately to severely active Crohn's disease (CD) in adults.1
SKYRIZI has
>95% coverage
in CD and UC2*
MEDICAL BENEFIT
PHARMACY BENEFIT
National commercial formulary coverage as of August 20252*
Any eligible, commercially insured patient who experiences a 5-day delay‡ in approval or is denied will be approved for a no‑charge shipment of SKYRIZI.§
PREFERRED FIRST-LINE TIM COVERAGE MEANS SKYRIZI IS AVAILABLE:
- For CD: No step through of biologic
- For UC: No step through of biologics and S1P receptor modulators
- At the lowest branded copay/coinsurance tier
*Coverage requirements and benefits designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies. Medical coverage may require a step edit depending on the plan’s management.
†SKYRIZI is on a preferred tier or otherwise has preferred status on the plan’s formulary.
‡For patients pending insurance approval of their medical benefit for the SKYRIZI IV induction dose.
§Eligibility criteria: Available to patients aged 63 or younger with commercial insurance coverage. Patients must have a valid prescription for SKYRIZI® (risankizumab-rzaa) for an FDA-approved indication and a denial of insurance coverage based on a prior authorization request on file along with a confirmation of appeal. For medical coverage, a delay of more than 5 business days or denial of treatment coverage from their insurance will be required. Continued eligibility for the program requires the submission of an appeal of the coverage denial every 180 days. Program provides for SKYRIZI® (risankizumab-rzaa) at no charge to patients for up to two years or until they receive insurance coverage approval, whichever occurs earlier, and is not contingent on purchase requirements of any kind. Program is not available to patients whose medications are reimbursed in whole or in part by Medicare, Medicaid, TRICARE, or any other federal or state program. Offer subject to change or discontinuance without notice. This is not health insurance and program does not guarantee insurance coverage. No claims for payment may be submitted to any third party for product dispensed by program. Limitations may apply.
TIM=targeted immunomodulator.
FIND AN INFUSION CENTER
You can find infusion centers for your SKYRIZI patients by using SKYRIZILocator.com
FIND AN INFUSION CENTER
You can find infusion centers for your SKYRIZI patients by using SKYRIZILocator.com
THREE REASONS TO ENROLL YOUR PATIENTS IN SKYRIZI COMPLETE
One person supporting patient access and reimbursement for your office

Patient access and education¶ through two treatment phases
Three potential ways to save

Commercially insured, eligible patients may pay as little as $0 per treatment. See savings program terms and conditions in footnotes.||
¶Patient access and education include processes and information around patient coverage through the benefits verification (BV) and prior authorization (PA) processes for both the infusion and on-body injector (OBI).
#Nurse Ambassadors are provided by AbbVie and do not provide medical advice or work under the direction of the prescribing health care professional (HCP). They are trained to direct patients to speak with their HCP about any treatment-related questions, including further referrals.
||Eligibility: Available to patients with commercial insurance coverage for SKYRIZI® (risankizumab-rzaa) who meet eligibility criteria. This co-pay assistance program is not available to patients receiving prescription reimbursement under any federal, state, or government-funded insurance programs (for example, Medicare [including Part D], Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs) or where prohibited by law. Offer subject to change or termination without notice. Restrictions, including monthly maximums, may apply. This is not health insurance. For full Terms and Conditions, visit www.SKYRIZICDSavingsCard.com or call 1.866.SKYRIZI for additional information. To learn about AbbVie's privacy practices and your privacy choices, visit https://abbv.ie/corpprivacy.
When your office or infusion center enrolls patients, Skyrizi Complete can also provide:
- Patient savings card information
- Proactive Field Reimbursement Manager (FRM) support
- Proactive plan benefits verification conducted
- Proactive bridge support for delays in prior authorization approvals
- Nurse ambassador

Enroll your patients in Skyrizi Complete for personalized support for your patients
Healthcare Provider (HCP) Office Enrollment
Infusion Site Enrollment