*Advanced therapy in Crohn's is defined as biologics.
The IL-23 inhibitor from AbbVie indicated for the treatment of moderately to severely active Crohn's disease (CD) in adults.1
Endoscopic and Symptom Control
ENDOSCOPIC RESPONSE AND CLINICAL REMISSION ENDPOINTS MET AT WEEKS 12 AND 52 WITH DATA OBSERVED AT WEEK 1521-3

FIRST IL-23 SPECIFIC INHIBITOR IN CROHN'S DISEASE1
IL-23=interleukin-23
ENDOSCOPIC AND SYMPTOM CONTROL ACROSS CO-PRIMARY ENDPOINTS1
†Advanced therapy in Crohn's is defined as biologics.
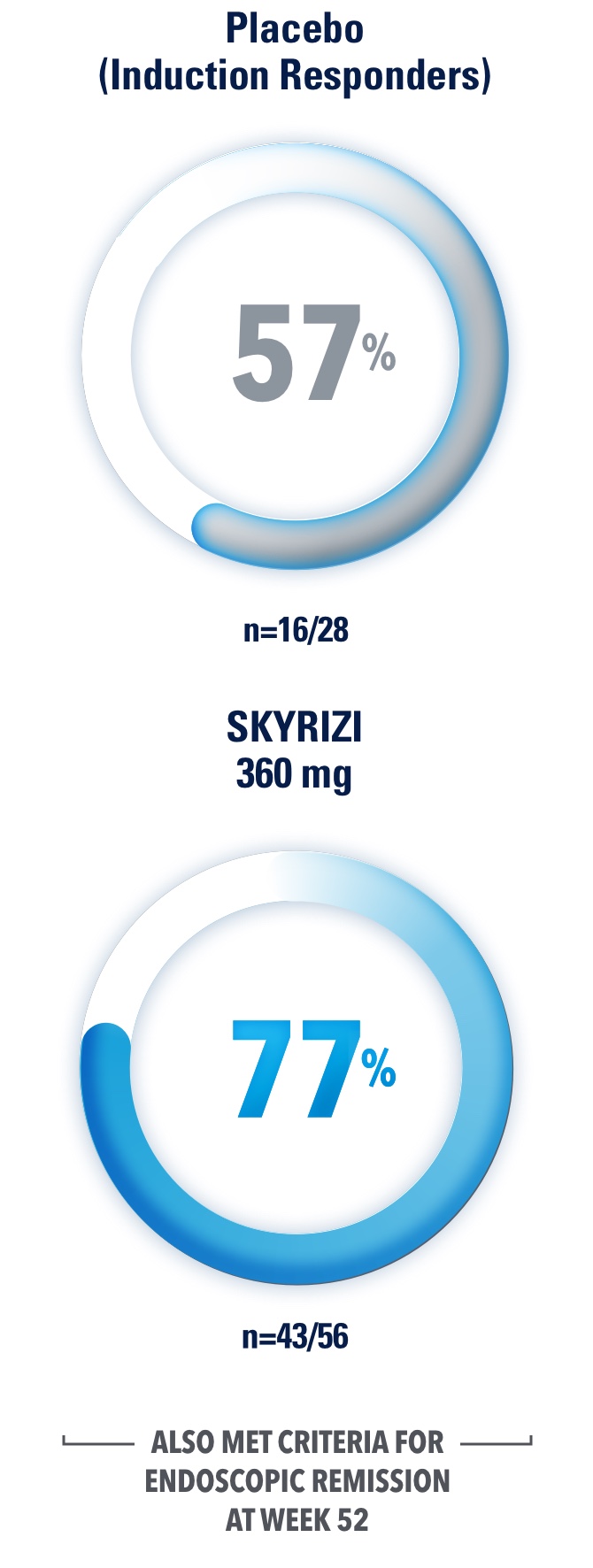
Placebo (Induction Responders): Patients who achieved CDAI clinical response (CR‑100)‖ to SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study.
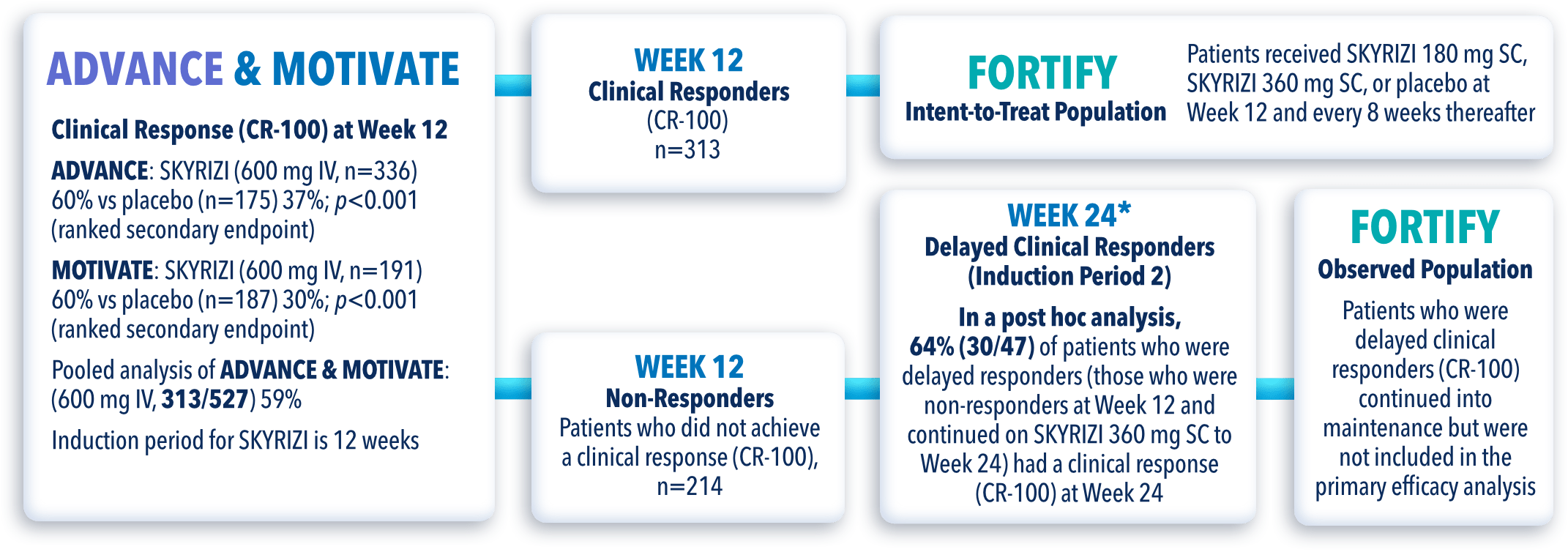
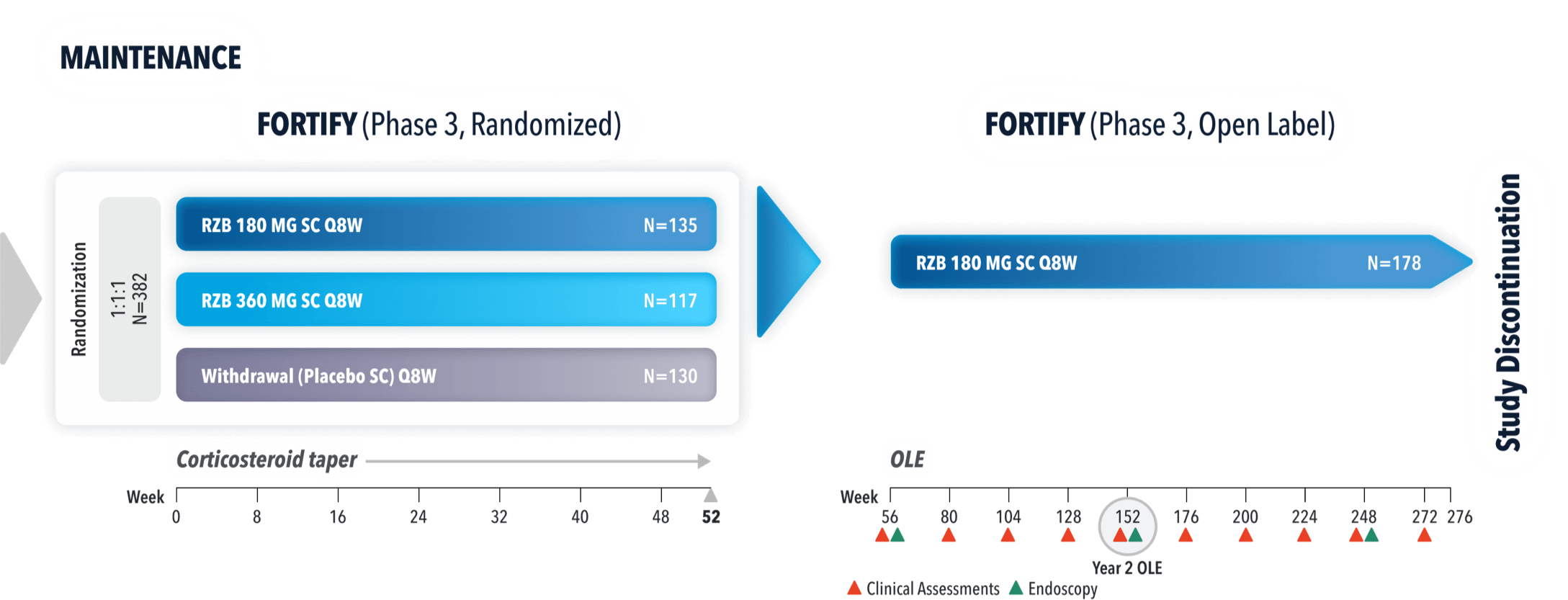
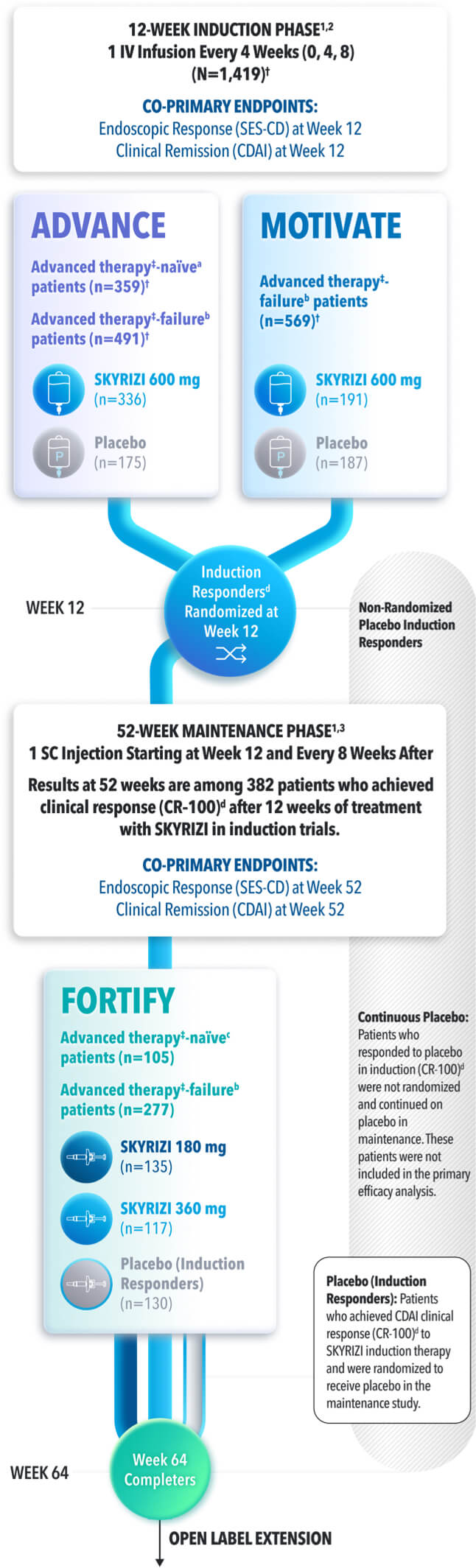
STUDY DESIGNS
ADVANCE (N=850) and MOTIVATE (N=569) Induction studies were 12-week, randomized, double-blind, placebo-controlled studies that evaluated the efficacy and safety of SKYRIZI in patients with moderately to severely active Crohn’s disease§ who demonstrated prior treatment failure to conventional and/or biologic treatment.3 Patients received an IV infusion of SKYRIZI 600 mg (recommended dose), risankizumab-rzaa 1200 mg|| or placebo at Weeks 0, 4, and 8.1
FORTIFY (N=382) Maintenance study was a 52-week study that evaluated the efficacy and safety of SKYRIZI in patients who achieved clinical response (decrease in CDAI ≥100)‡ from SKYRIZI induction in the ADVANCE and MOTIVATE studies. Patients were randomized to SKYRIZI 180 mg SC, SKYRIZI 360 mg SC, or placebo at Week 12 and every 8 weeks thereafter.1
FORTIFY OPEN-LABEL EXTENSION (OLE) STUDY DESIGN
FORTIFY-OLE is an ongoing, multicenter, open-label extension study that evaluated long-term efficacy and safety of SKYRIZI that included patients from the FORTIFY maintenance study. Patients in the FORTIFY maintenance study achieved clinical response with SKYRIZI in the ADVANCE and MOTIVATE induction studies. This study population for FORTIFY-OLE is a sub analysis that includes all randomized subjects who had a baseline eligibility SES-CD of ≥6 (≥4 for isolated ileal disease), received 600 mg IV SKYRIZI for 12 weeks in the induction studies, have received at least one dose of SKYRIZI 180 mg or 360 mg SC in the Phase 3 maintenance study FORTIFY, and at least one dose of SKYRIZI 180 mg SC in the FORTIFY OLE study.
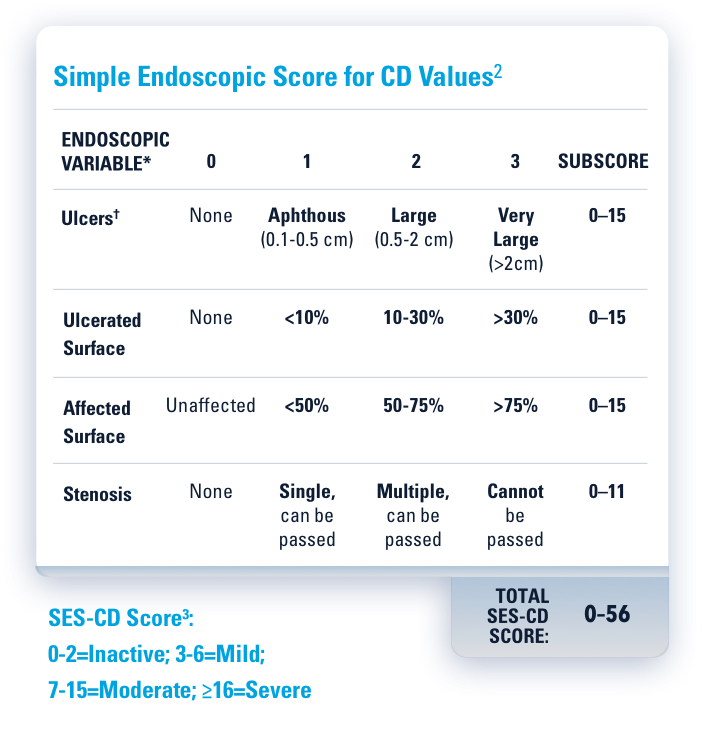
ENDOSCOPIC CONTROL: VISIBLE MUCOSAL IMPROVEMENT
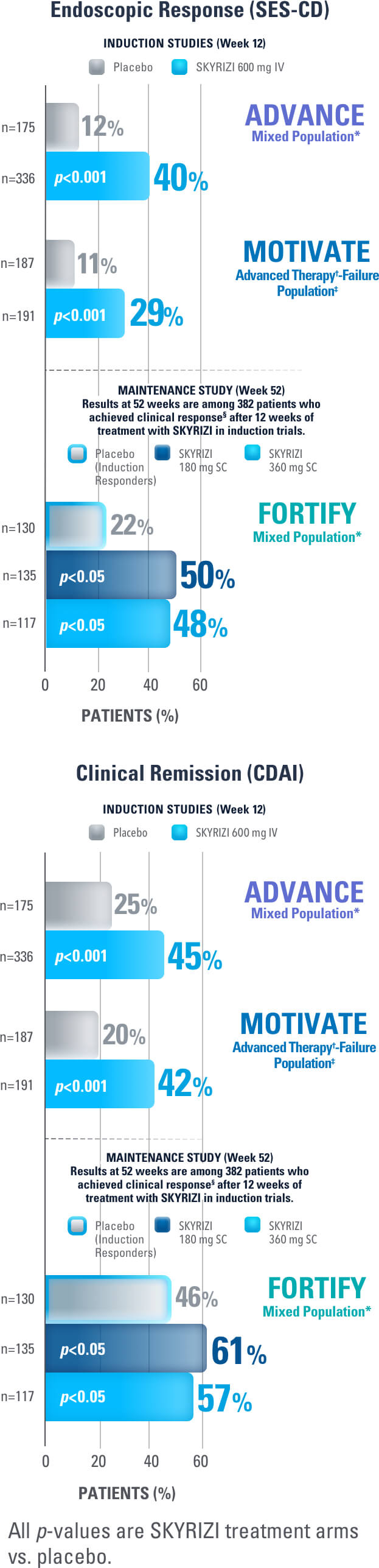
SKYRIZI Achieved its Co-Primary Endpoint of Endoscopic Response at Week 12 and Week 521,3
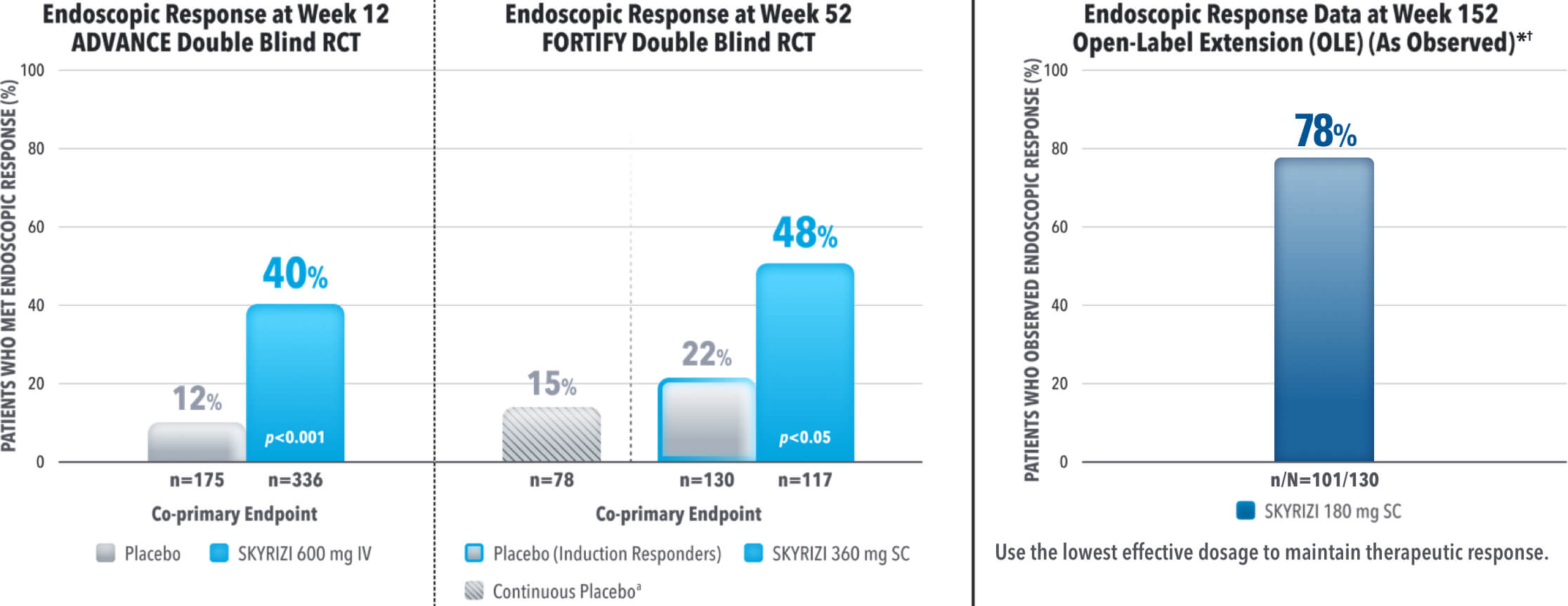
Results at 52 weeks are among 382 patients who achieved clinical response‡ after 12 weeks of treatment with SKYRIZI in induction trials.1
aContinuous placebo data not intended for direct comparison.
*OLE Limitations: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
†AO Disclosure: In an as observed (AO) analysis, missing visit data was excluded from calculations for that visit, which may increase the percent of responders. All observed data was used regardless of premature discontinuation of study drug, or initiation of concomitant medication. The same patient may not have a response at each timepoint.
IMPROVE PATIENTS’ POSSIBILITY OF ENDOSCOPIC REMISSION
SKYRIZI SECONDARY ENDPOINT OF ENDOSCOPIC REMISSION RATES AT WEEK 12 AND 521,3
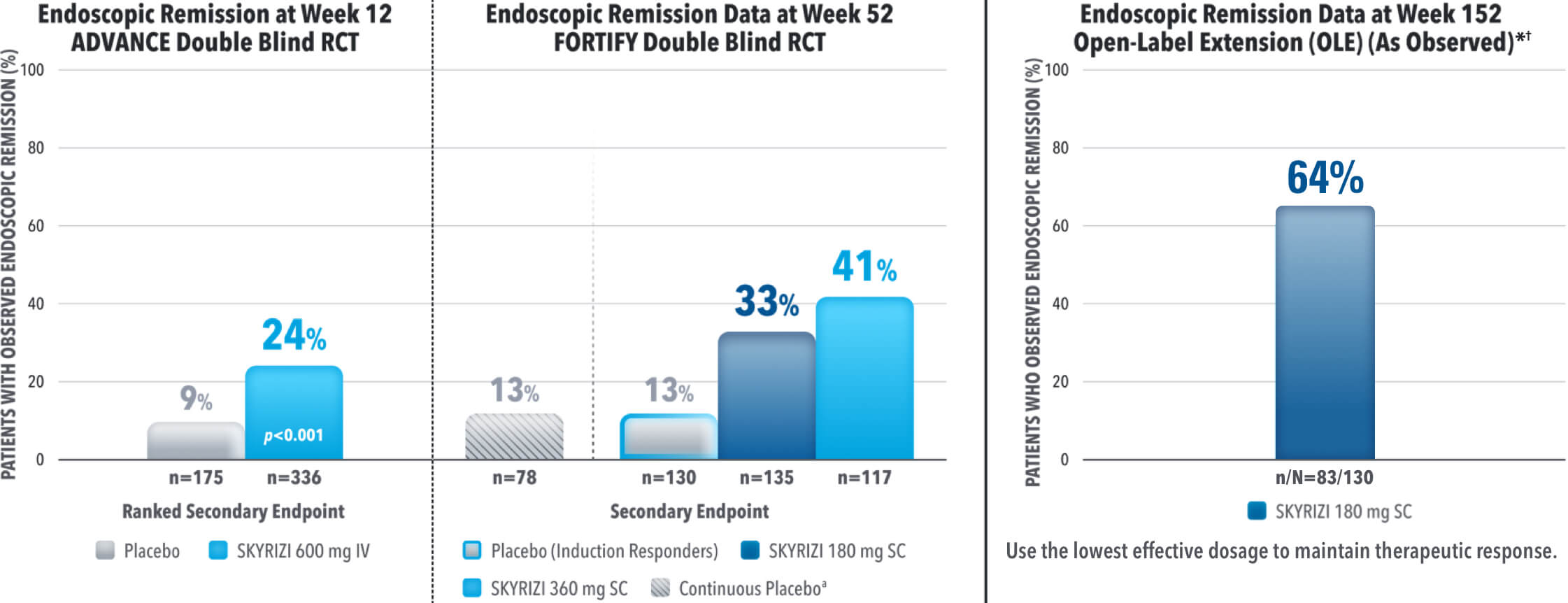
DATA LIMITATIONS: Endoscopic remission at Week 52 was not statistically significant under the pre‑specified multiple testing procedure.
Results at 52 weeks are among 382 patients who achieved clinical response‡ after 12 weeks of treatment with SKYRIZI in induction trials.
aContinuous placebo data not intended for direct comparison.
*OLE Limitations: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
†AO Disclosure: In an as observed (AO) analysis, missing visit data was excluded from calculations for that visit, which may increase the percent of responders. All observed data was used regardless of premature discontinuation of study drug, or initiation of concomitant medication. The same patient may not have a response at each timepoint.
EARLY AND DURABLE SYMPTOM CONTROL
SKYRIZI ACHIEVED ITS CO-PRIMARY ENDPOINT OF CLINICAL REMISSION AT WEEK 12 AND WEEK 521-3
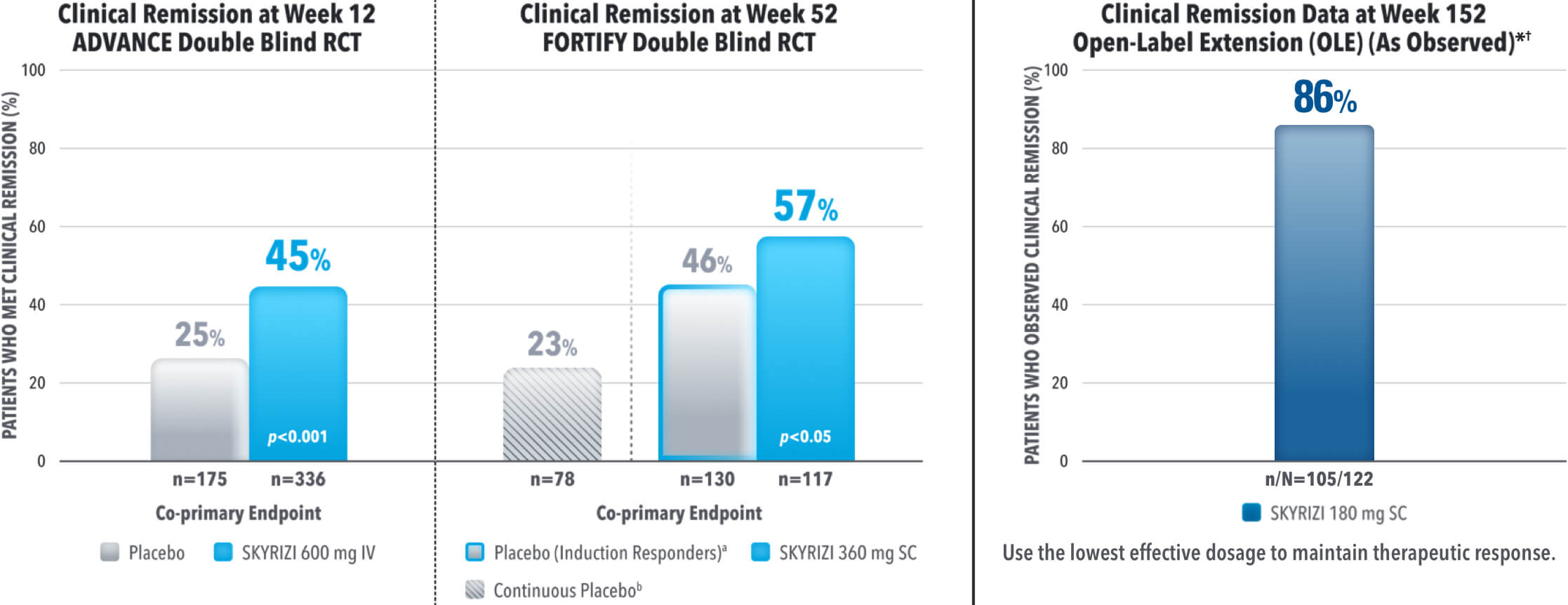
aThe induction-only group consisted of subjects who achieved clinical response (CR-100) to SKYRIZI induction therapy and were randomized to receive placebo in FORTIFY. The mean half-life of SKYRIZI is approximately 21 days for patients with Crohn's which may have contributed to these rates.
Results at 52 weeks are among 382 patients who achieved clinical response‡ after 12 weeks of treatment with SKYRIZI in induction trials.
bContinuous placebo data not intended for direct comparison.
*OLE Limitations: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
†AO Disclosure: In an as observed (AO) analysis, missing visit data was excluded from calculations for that visit, which may increase the percent of responders. All observed data was used regardless of premature discontinuation of study drug, or initiation of concomitant medication. The same patient may not have a response at each timepoint.
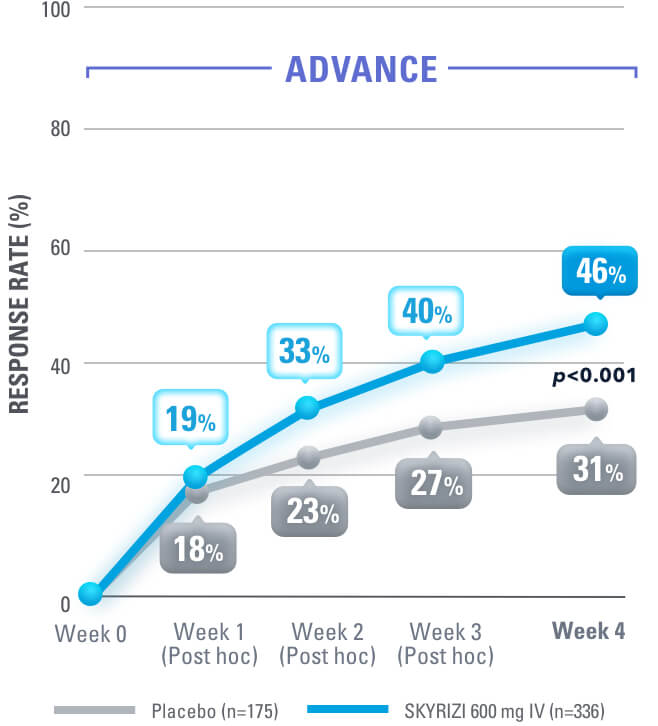
SYMPTOM RELIEF AS EARLY AS WEEK 4:
Clinical Response (CDAI CR-100) at Weeks 4, 8, 121,4
Data Limitations: Data not labeled as a ranked secondary endpoint were prespecified nonranked endpoints not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
Clinical Response: Reduction of CDAI score ≥100 points from baseline.
Data Limitations: Data not labeled as a ranked secondary endpoint were prespecified nonranked endpoints not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
Clinical Response: Reduction of CDAI score ≥100 points from baseline.
at week 12 in induction studies1,4
Clinical Response is defined as a reduction of CDAI score ≥100 points from baseline.
WHAT DID IMPROVEMENT LOOK LIKE IN A REAL BIO-NAÏVE PATIENT?
SEE THESE RESULTS IN AN ACTUAL PATIENT CASE WITH SKYRIZI5
- Clinical Trial Patient Profile: Age: 40's
- Sex: Female
- Disease Duration: 2.1 Years
- Disease Severity: Moderate to Severe
Drug history: Bio-Naïve Patient received SKYRIZI 600 mg IV (Induction) and 180 mg SC (Maintenance)
Corticosteroid use at baseline*
*Corticosteriods were tapered starting at Week 0 of FORTIFY.
Actual Patient Presenting With Remission at Weeks 12 and 52
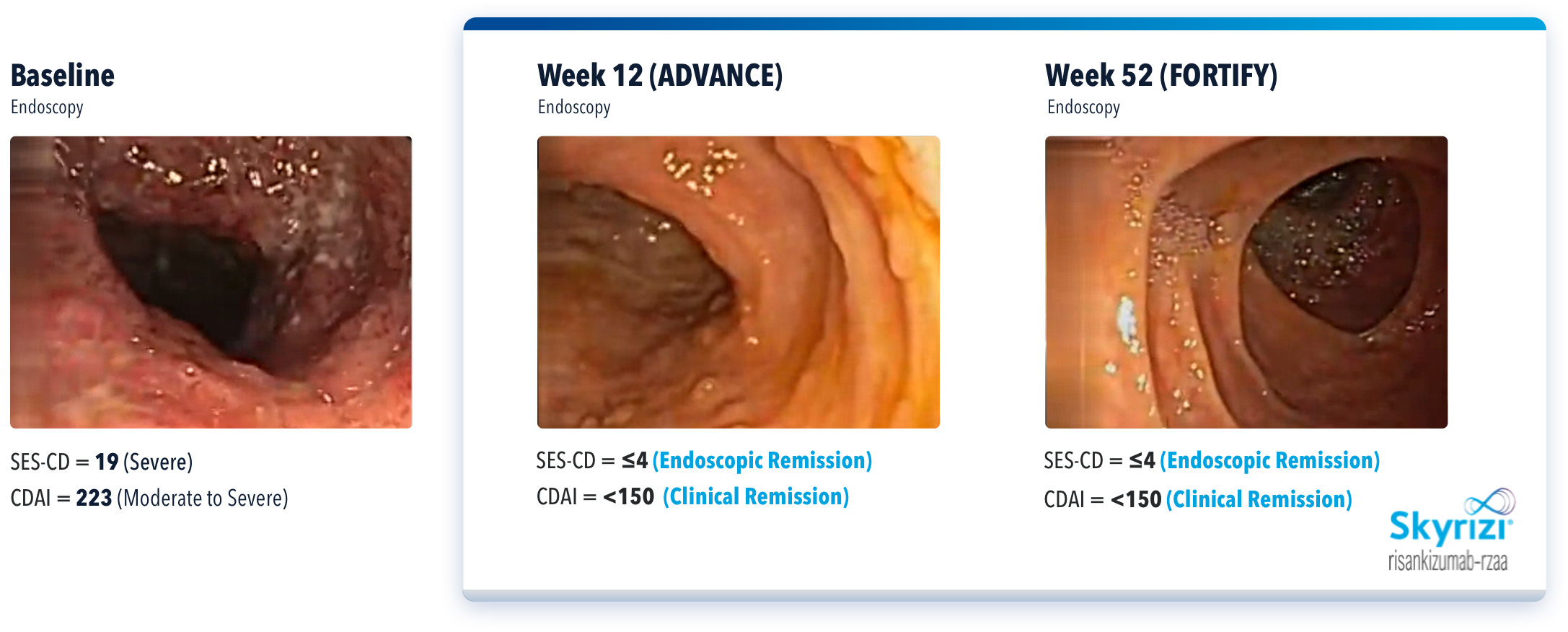
THIS IMAGE REPRESENTS AN INDIVIDUAL PATIENT WHO HAD ENDOSCOPIC REMISSION AT WEEK 52. IN THE FORTIFY MAINTENANCE CLINICAL TRIAL, ENDOSCOPIC REMISSION DATA AT WEEK 52 WAS NOT STATISTICALLY SIGNIFICANT UNDER THE PRE-SPECIFIED MULTIPLE TESTING PROCEDURE.
IMAGES ARE FROM A CLINICAL TRIAL PATIENT TREATED WITH SKYRIZI. INDIVIDUAL RESULTS MAY VARY.