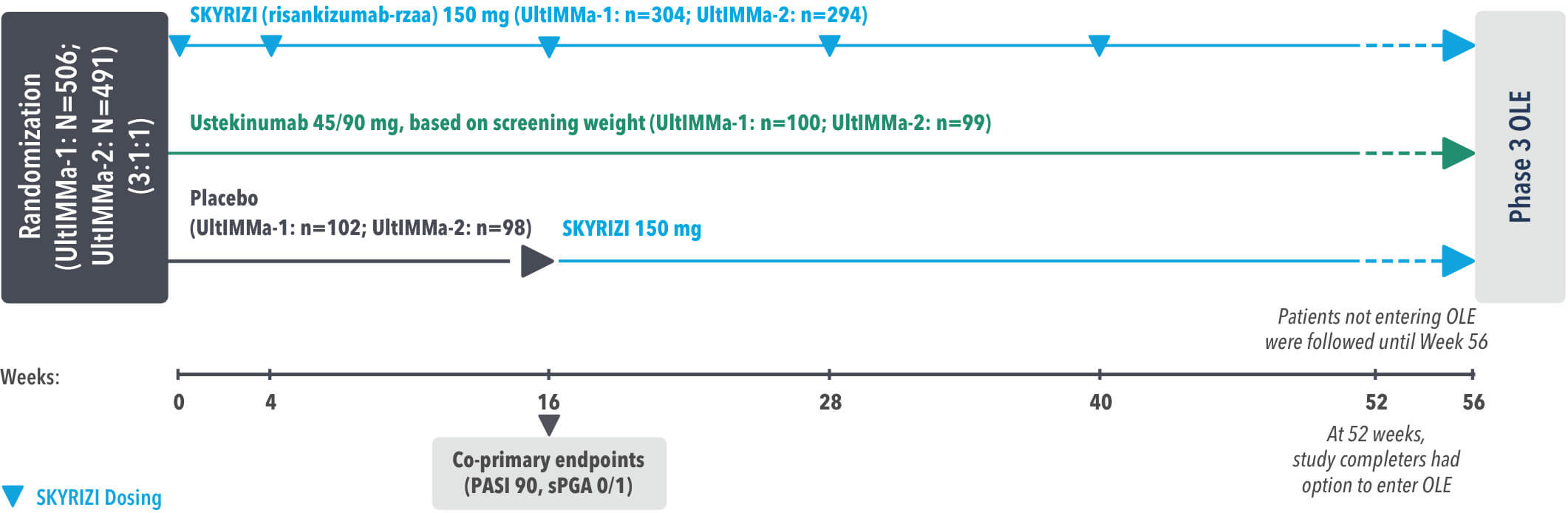
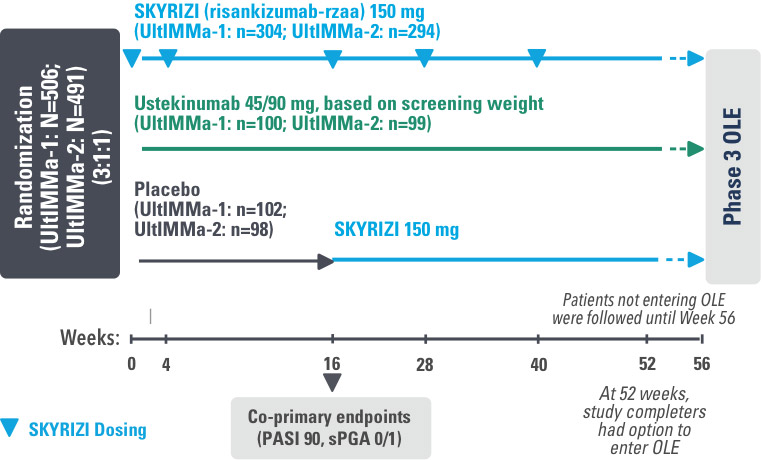
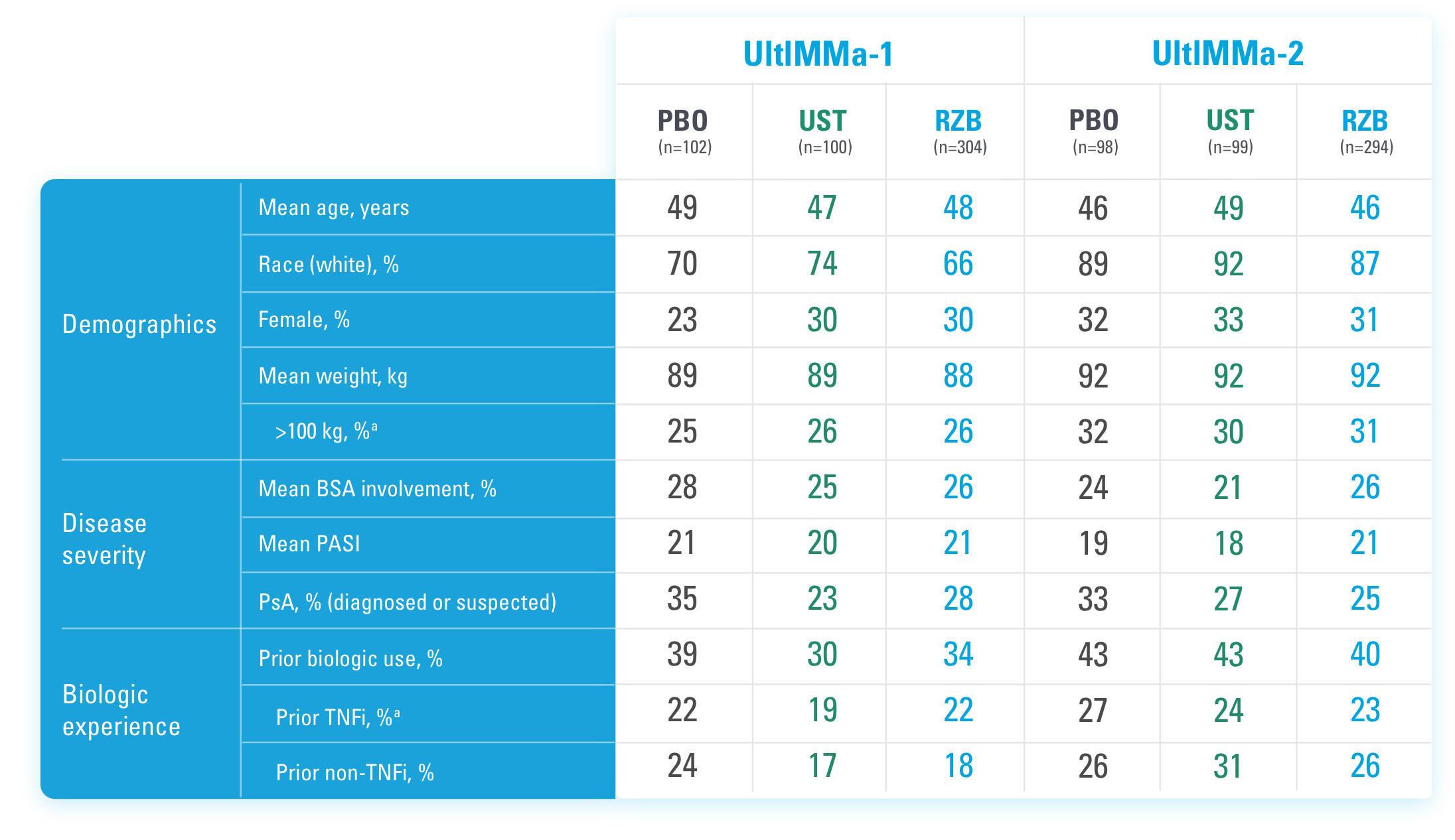
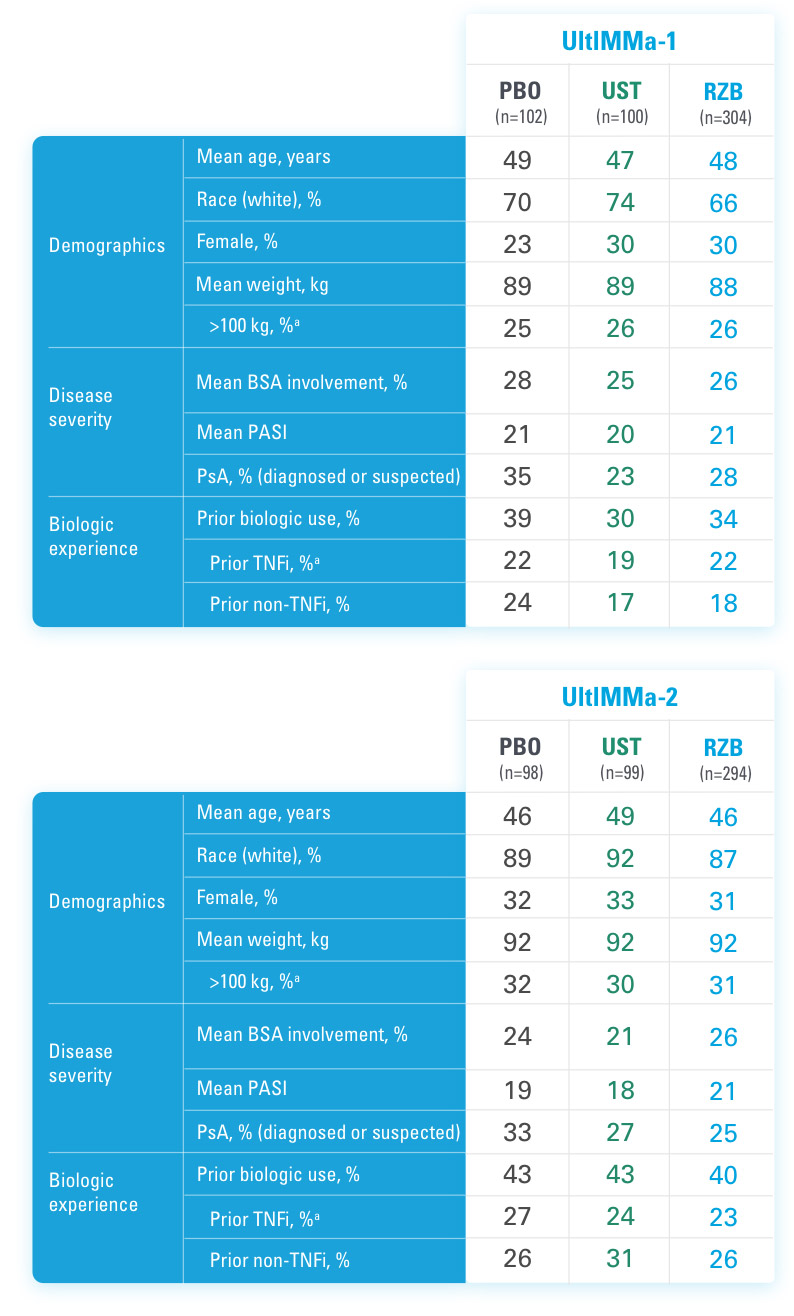
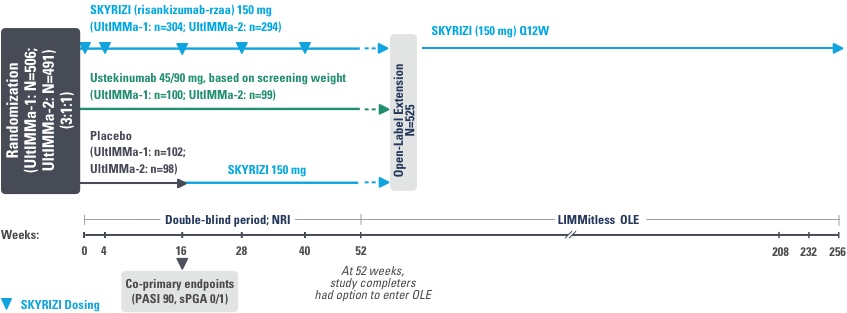
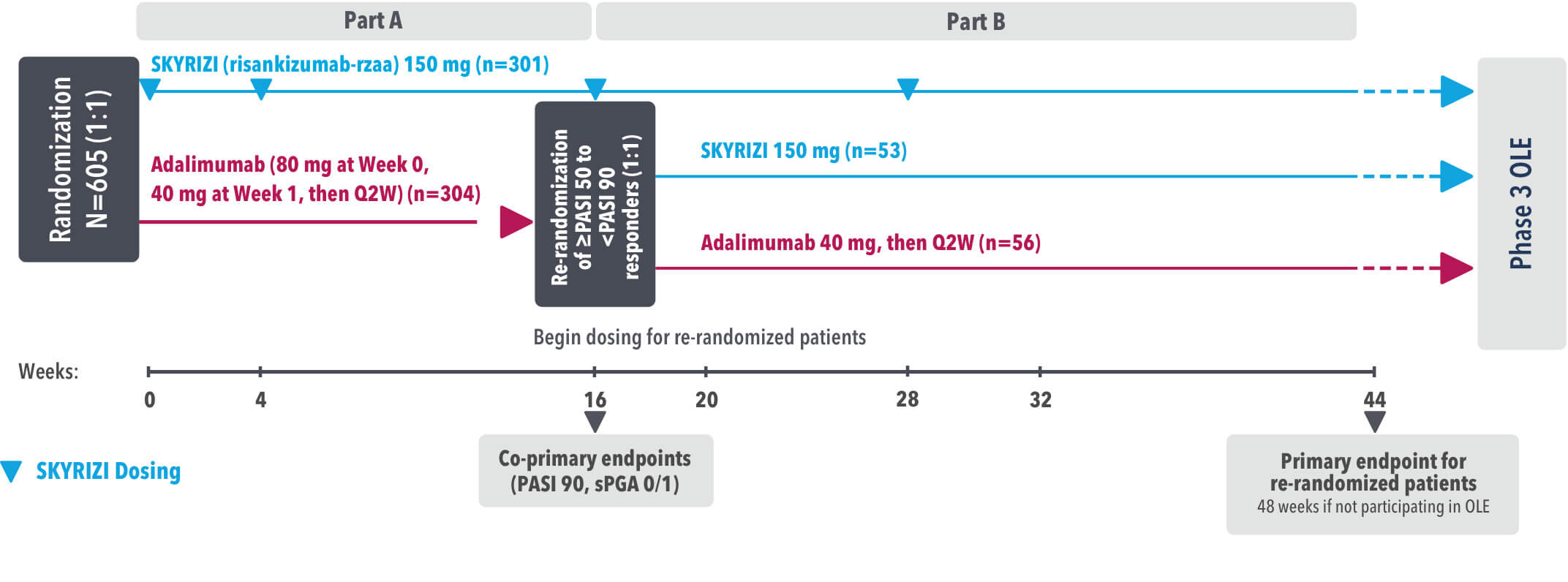
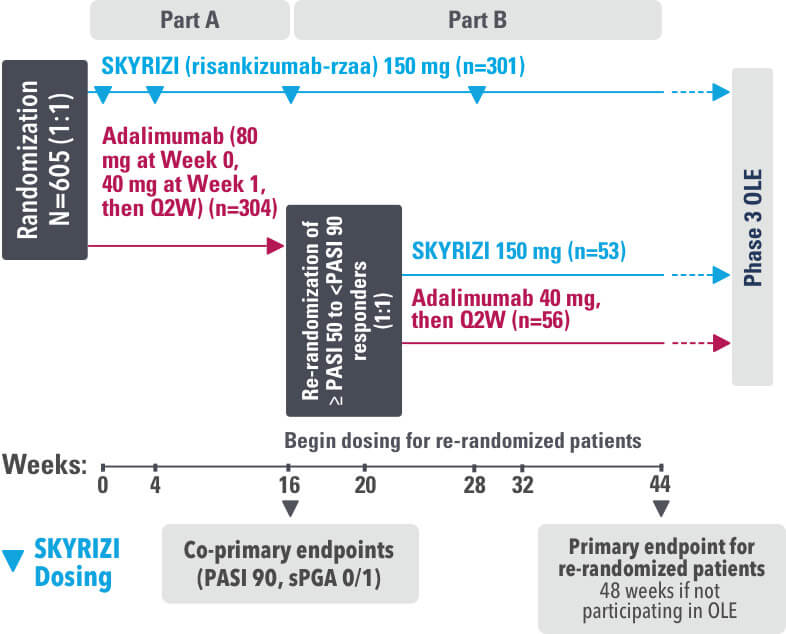
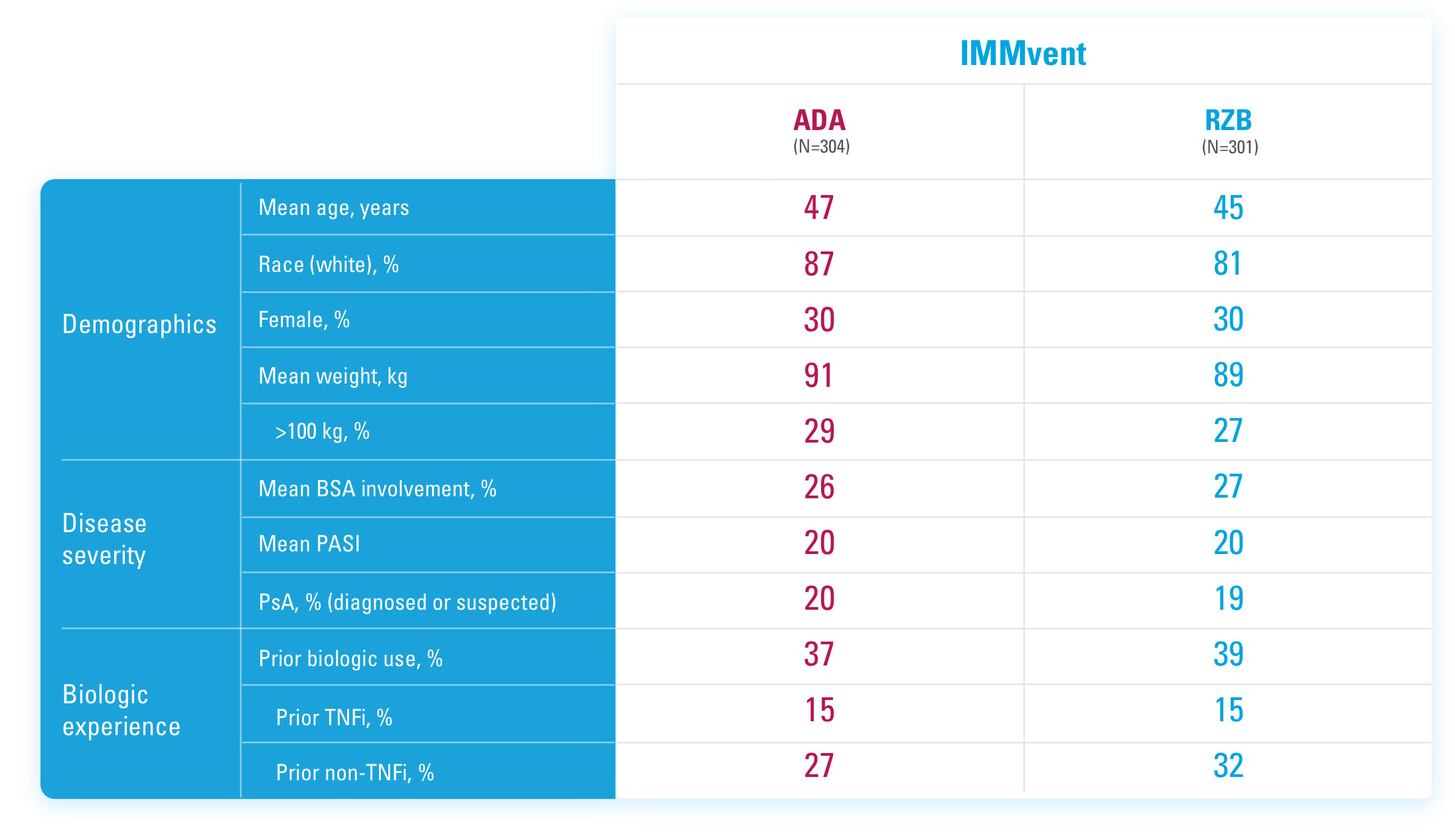
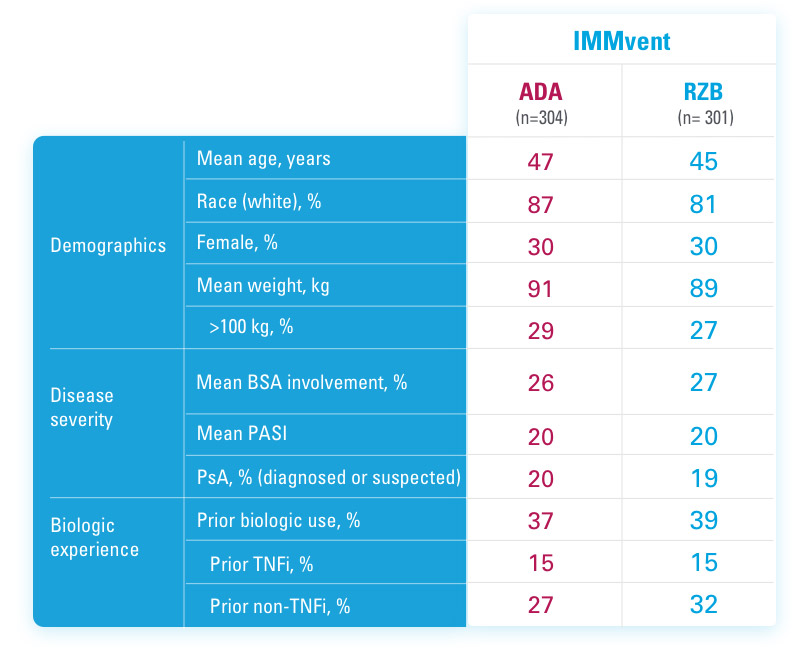
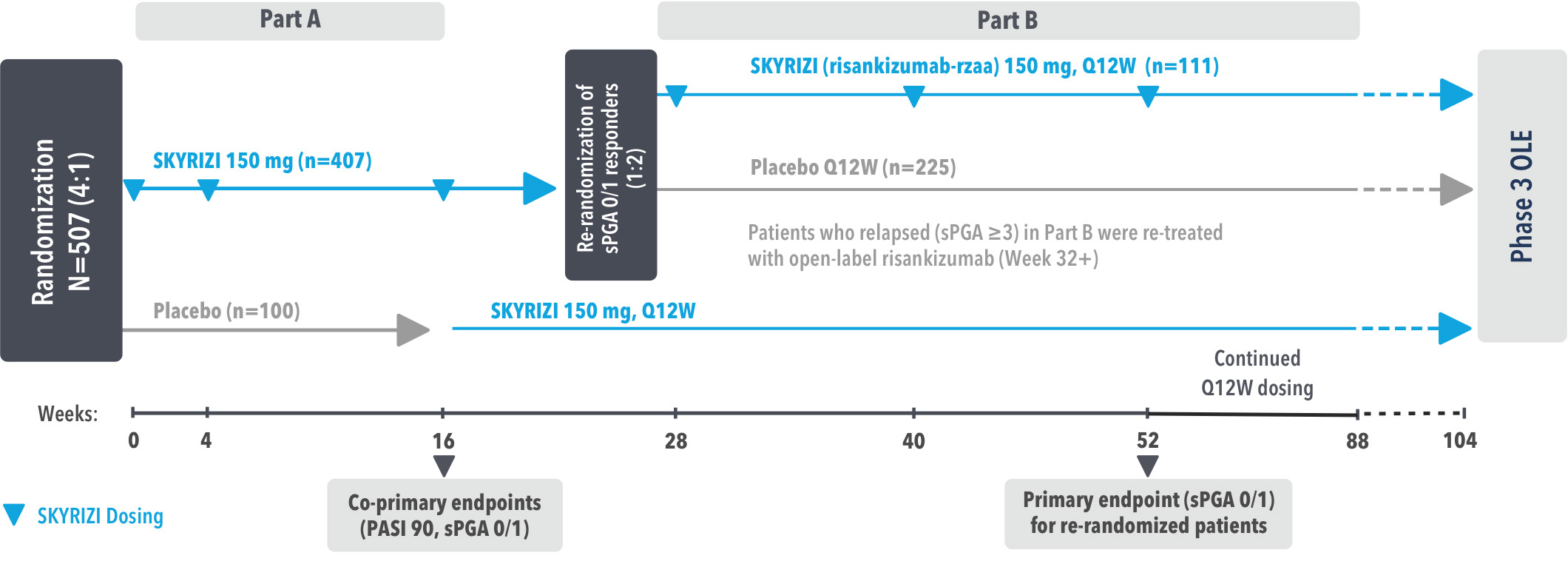
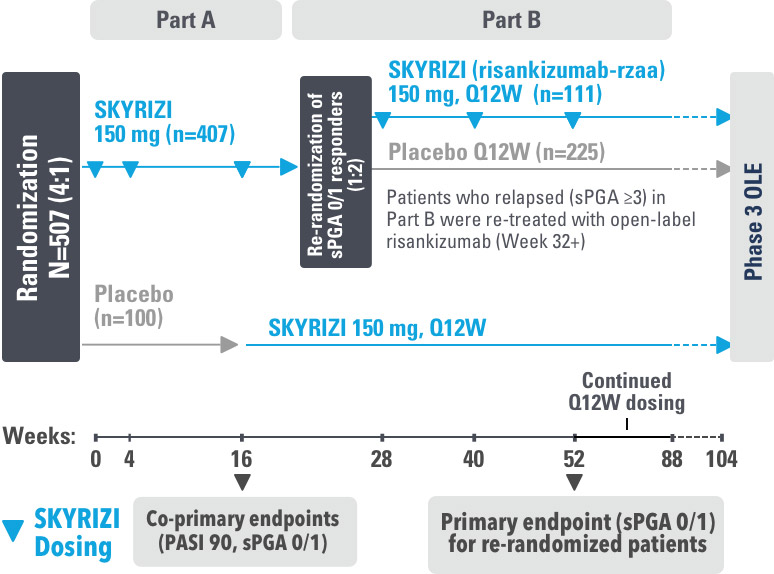
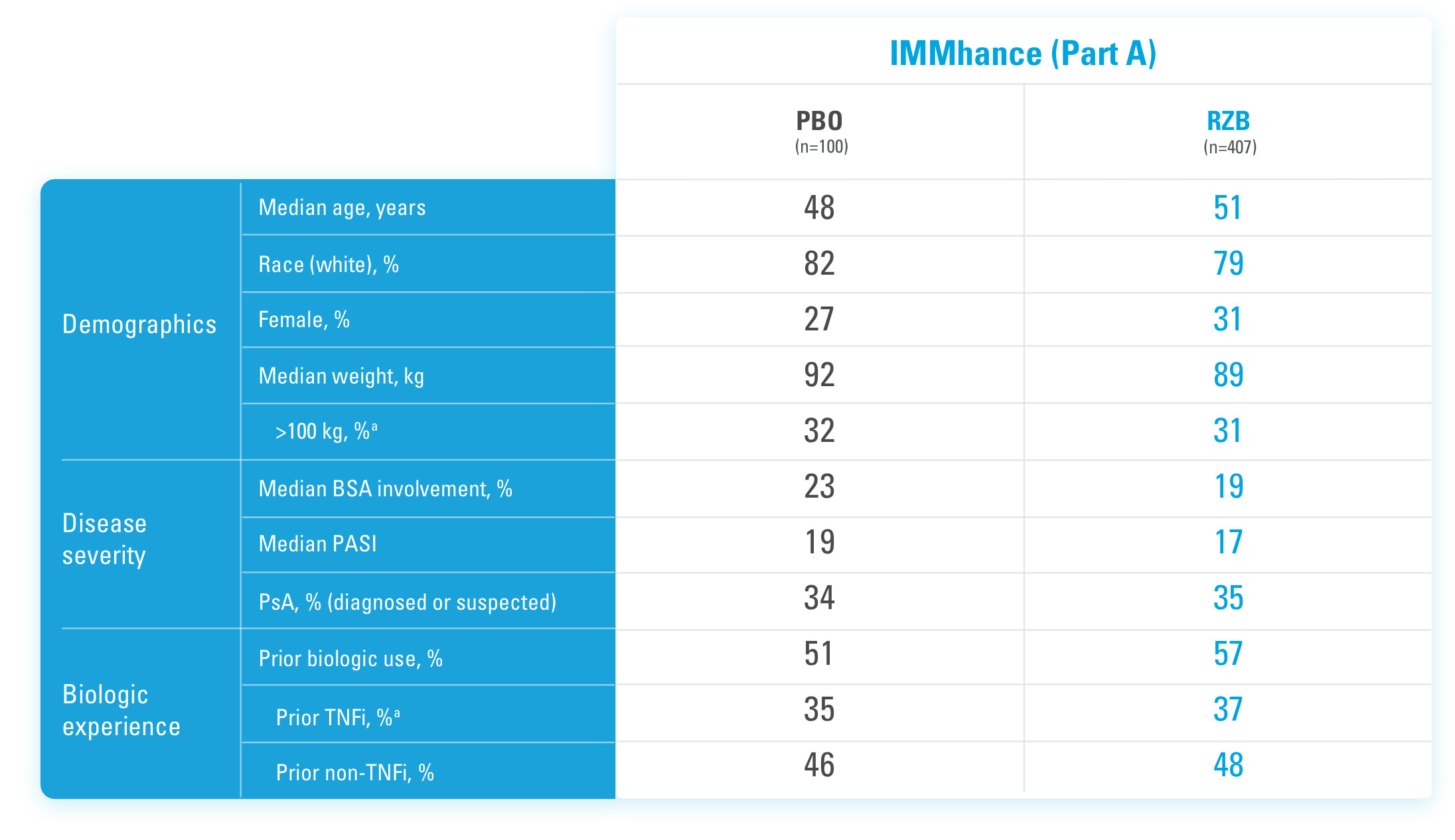
SYRINGE INJECTION VIDEO
DISCLAIMER
SUPER: This demonstration is a guide to injecting with the SKYRIZI prefilled syringe. Watch it and also read the entire SKYRIZI Instructions for Use leaflet, inserted with your medication package. Don’t try to inject SKYRIZI until your doctor has decided you can, and you’ve been shown the right way to inject. Please see Instructions for Use and Important Safety Information within the website. Please see accompanying full Prescribing Information, including Medication Guide, and discuss with your doctor.
AUDIO: This demonstration is a guide to injecting with the SKYRIZI prefilled syringe. Watch it and also read the entire SKYRIZI Instructions for Use leaflet, inserted with your medication package. Don’t try to inject SKYRIZI until your doctor has decided you can, and you’ve been shown the right way to inject
VIDEO: A GUIDE TO INJECTING SKYRIZI
VIDEO: We see the patient comfortably seated on a couch in their living room. Patient looks into camera and introduces himself.
SUPER: MAYA
AUDIO: Hey there! I’m Maya and I’m here to walk you through how to inject yourself with SKYRIZI. Now, if it’s your first time, don’t worry! If it’s not your first time, I hope you pick up some new tips while you’re here.
VIDEO: Patient speaks to camera, points to “Bryan (Nurse Ambassador)” contact on phone.
SUPER:
Don’t have a Nurse Ambassador?*
Call 1.866.SKYRIZI or visit www.SKYRIZI.com
*Nurse Ambassadors are provided by AbbVie and do not work under the direction of your health care professional (HCP) or give medical advice. They are trained to direct patients to their HCP for treatment-related advice, including further referrals.
AUDIO: Now, I’ve done this a few times by myself, but my doctor originally walked me through how to inject, and I called Bryan, who’s my Nurse Ambassador, when I first started injecting on my own at home.
VIDEO: Patient stands and walks out of frame. INJECTION PREP
VIDEO: Patient walks to fridge, pulls out SKYRIZI package, shows to camera, places on counter. SUPERS rotate through on screen.
SUPER: Do not warm SKYRIZI in any other way (for example, do not warm it in a microwave or in hot water).
Do not use SKYRIZI if liquid has been frozen even if it has been thawed.
15 to 30 minutes
Keep SKYRIZI in the original carton to protect from light until time to use.
AUDIO: Alright, let’s get started. So, when my SKYRIZI comes in the mail, it goes straight in the fridge. Now, before I inject, I let it sit out for 15 to 30 minutes, out of direct sunlight, to get to room temperature. And while I wait, I try to relax.
VIDEO: Patient sits down on couch with items needed on the table in front of her, SUPERS rotate through on screen. Supply names animate on screen next to each item, as she speaks their names.
SUPER:
Do not shake SKYRIZI.
Do not use SKYRIZI if the syringe has been dropped or damaged.
Do not use SKYRIZI if package perforations are broken. Return product to the pharmacy.
Do not remove the needle cover until right before injection.
Do not use SKYRIZI if expiration date (EXP) has passed.
SKYRIZI syringe, with cardboard sleeve removed, alcohol swab not included in your package, Instructions for Use, cotton ball not included in your package, sharps container
AUDIO: Okay, that should be enough time. So, once my hands are clean, I like to put out everything that I’ll need. So, I have my SKYRIZI syringe. I have an alcohol swab, which I got from my local pharmacy. I also have the Instructions for Use, just in case. And I also like to grab a cotton ball (I had some right in my medicine cabinet) and then a sharps container. Now, I like this spot because there’s a lot of space and I don’t have to leave my couch.
VIDEO: HOW TO INJECT step-by-step
VIDEO: Patient points to 4 Ps graphic on package.
AUDIO: So, it helps me to break down the injection process into 4 simple steps: the 4 Ps. And you can see them here on the package. We have: pick, prepare, pinch, push. Okay?
SUPER: PICK the injection site
AUDIO: So, first, I pick my injection site. So, I’ll go with my left thigh. But, you can also pick your right thigh or your lower stomach.
ANIMATION: 2 inches graphic animates.
SUPER: 2 INCHES
AUDIO: But, if you choose your stomach, make sure to inject at least 2 inches away from your belly button. Okay?
VIDEO: Patient demonstrates step.
AUDIO: So, you want to grab your alcohol swab to clean your skin in a circular motion, like this okay, and let it dry.
VIDEO: Patient holds syringe with needle pointing down, removes cap to check liquid, shows to camera. SUPERS rotate through on screen.
SUPER:
Do not touch or blow on the injection site after it is cleaned. Allow the skin to dry before injecting.
Do not inject through clothes.
Do not inject into skin that is sore, bruised, red, hard, scarred, or has stretch marks.
Do not use if the liquid is cloudy or contains flakes or large particles.
AUDIO: Now, I hold my syringe with the needle facing down, and I check the liquid inside to make sure that everything looks okay. It should be clear to slightly yellow, which it is. See that? There are a few bubbles in there, but that’s normal. Looks like we’re good to go!
VIDEO: Patient removes the needle cover by holding the syringe in one hand and gently pulling the needle cover straight off with the other hand.
SUPER:
PREPARE the syringe
Do not hold or pull plunger when removing the needle cover.
Do not touch the needle with your fingers or let the needle touch anything.
AUDIO: Alright, so I hold my syringe in one hand, and I take the needle cover off with the other hand, okay? Like this, and then you want to throw it away. You see? You may see a drop of liquid at the end of the needle, which is normal, okay?
VIDEO: Patient demonstrates steps.
SUPER:
PINCH the skin
AUDIO: So, I’ve got my syringe in one hand, and with the other hand, I’m gently pinching the area of my cleaned skin to hold firmly.
VIDEO: Patient demonstrates step.
SUPER:
PUSH the plunger in
45°
AUDIO: Now I’ll inject the needle into my skin at about a 45-degree angle using a quick, short movement — like this. Now, holding the angle steady, I slowly push the plunger in all the way until all of the liquid is injected, and the syringe is empty. There we go.
VIDEO: Patient demonstrates step.
AUDIO: Now, I pull the needle out of my skin while keeping the syringe at the same angle, like this. I release the plunger to allow the syringe to move up.
VIDEO: Patient points to needle guard on syringe.
AUDIO: Until the entire needle is covered up by the needle guard. See how that works? The needle guard won’t come back down unless all the liquid has been injected.
VIDEO: Patient demonstrates step. SUPERS rotate through on screen.
SUPER:
Press a cotton ball or gauze pad over the injection site and hold for 10 seconds.
Do not rub the injection site. You may have slight bleeding. This is normal.
AUDIO: Alright, so now, I press my cotton ball where I just injected, and that’s it!
VIDEO: Patient demonstrates step.
SUPER:
Do not rub the injection site. You may have slight bleeding. This is normal.
AUDIO: I drop my used syringe in one of these handy sharps disposal containers. Voilá – safe and sound.
VIDEO: Patient places the sharps container on closet shelf.
AUDIO: Now I’ll just put it back out of reach. Just like that, another injection complete.
VIDEO: HELPFUL TIPS for self-injecting
ANIMATION/SUPER:
PICK the injection site
PREPARE the syringe
PINCH the skin
PUSH the plunger in
Text animates on screen for each step.
AUDIO: So, to recap, I prepare myself and my space. I pick an injection site, prepare the syringe, pinch the skin, push the plunger in.
VIDEO: Patient demonstrates step.
AUDIO: And safely discard the syringe. That’s the whole injection process!
VIDEO: Patient speaks to camera.
AUDIO: Now, it seemed tricky at first, but I got it down soon enough. And Bryan, my Nurse Ambassador, he recommended I create a routine — injecting at the same time, same place — that sort of thing. I also do something nice for myself after each injection — maybe I’ll paint as a reward.
VIDEO: Patient holds up phone to camera.
AUDIO: Oh, and this Skyrizi Complete App has helped me so much.
VIDEO: Patient points to App on phone.
AUDIO: I mean I used it to log my first starter dose so I knew when to take my second starter dose 4 weeks later. You can set reminders for future doses, too.
VIDEO: Patient speaks to camera.
SUPER: 1 INJECTION 4x A YEAR
AUDIO: Since, after 2 starter doses, SKYRIZI is only 1 injection 4 times a year.
VIDEO: Patient points to phone.
App notification reminder appears on phone screen.
AUDIO: And when I get those reminders, I know to contact my specialty pharmacy to get my next injection on time.
VIDEO: Patient speaks to camera.
SUPER: Get sharps containers for no additional cost at www.SKYRIZI.com or call 1.866.SKYRIZI
AUDIO: Oh, and I can’t forget about the Skyrizi Complete sharps disposal and mail-back service.
ANIMATION: SUPER animates over sharps container
SUPER: Get sharps containers for no additional cost at www.SKYRIZI.com or call 1.866.SKYRIZI
FULL
AUDIO: Once my sharps container gets full, I request a new one that’s sent to me with a box to mail this one back. I signed up for the service online, and it doesn’t cost me anything!
VIDEO: Patient speaks to camera. SUPER animates on screen.
SUPER: CALL 1.866.SKYRIZI FOR 24/7 SUPPORT WITH SKYRIZI COMPLETE
AUDIO: I’m lucky to have people around to support me. I’ve got Skyrizi Complete on my team, too. Like Bryan, who’s such a good listener,
VIDEO: Patient picks up phone from table.
SUPER: CALL 1.866.SKYRIZI FOR 24/7 SUPPORT WITH SKYRIZI COMPLETE
AUDIO: and this App!
VIDEO: Patient speaks to camera.
AUDIO: There’s always someone to reach out to if you need help. You’ve got this.
VIDEO: SAFETY CONSIDERATIONS1
SKYRIZI may cause serious side effects, including:
Serious allergic reactions: Stop using SKYRIZI and get emergency medical help right away if you get any symptoms of a serious allergic reaction.
Infections: SKYRIZI may increase your risk of infections. Before starting treatment, your doctor should check you for infections and tuberculosis. Tell your doctor right away if you have an infection or symptoms of one.
Do not use SKYRIZI if you are allergic to risankizumab-rzaa or any of the ingredients in SKYRIZI.
Also, tell your doctor if you plan to or recently received a vaccine.
REFERENCE: 1. SKYRIZI [package insert]. North Chicago, IL: AbbVie, Inc.