Picture what improvement could look like
SEE ACTUAL PSORIASIS PATIENTS TREATED
WITH SKYRIZI (risankizumab-rzaa)2
Photo depicts moderate to severe plaque psoriasis patient with affected body surface area ≥10% at baseline, outside of clinical trials.
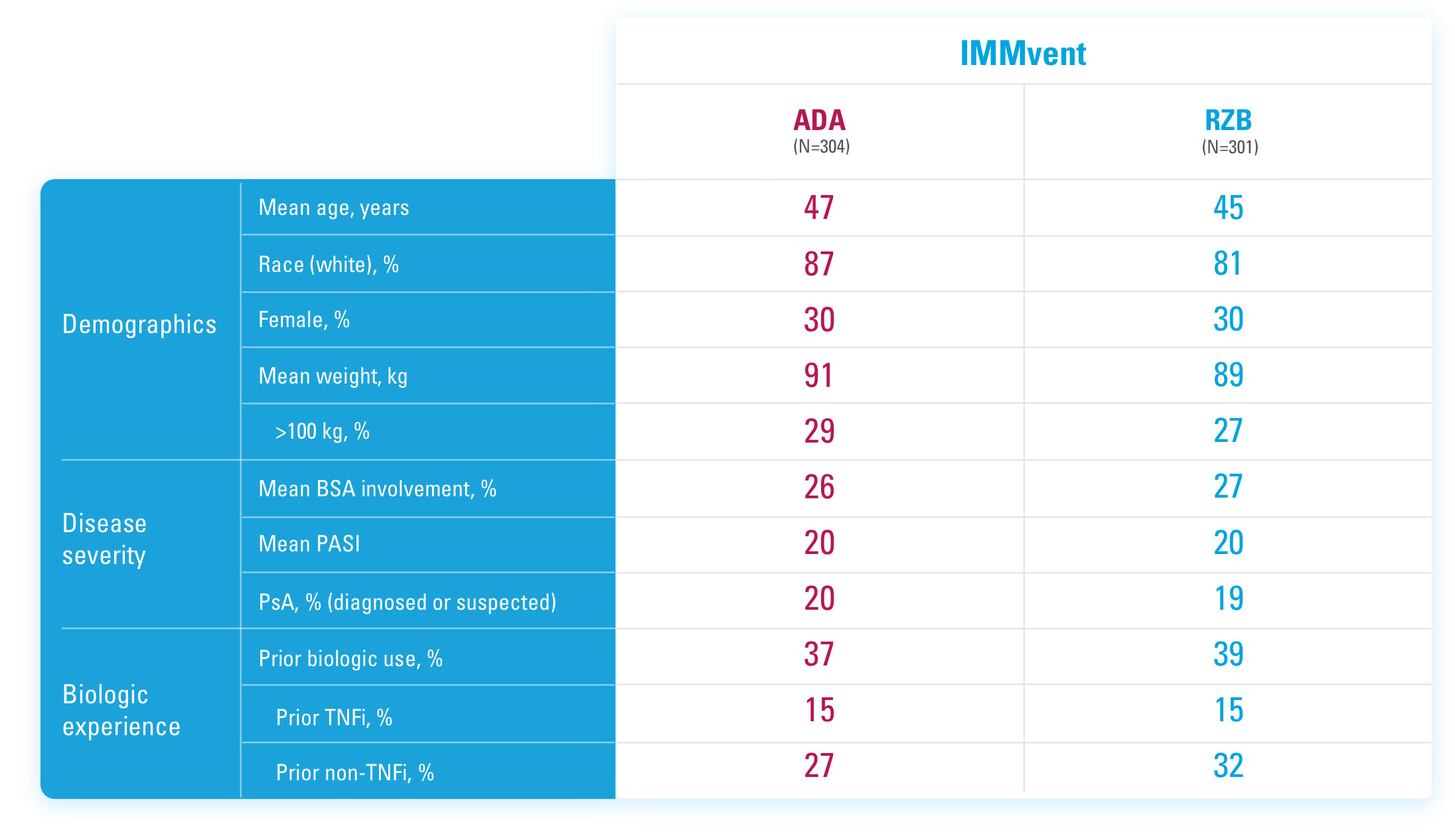
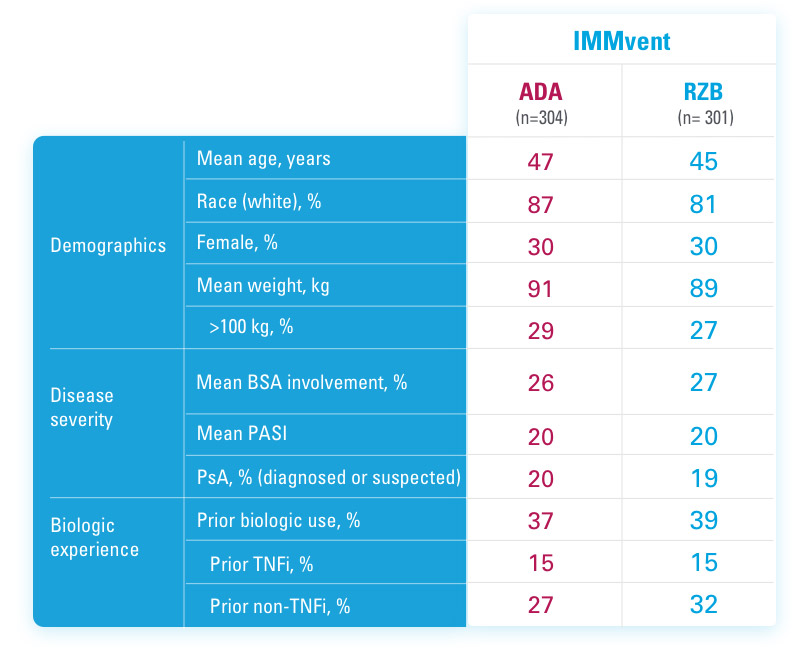
CO-PRIMARY ENDPOINTS IN UltIMMA-1 AND UltIMMA-2 (NRI)1,3
PASI 90 at Week 16
UltIMMa-1:
SKYRIZI 75% (229/304), placebo 5% (5/102)
UltIMMa-2:
SKYRIZI 75% (220/294), placebo 2% (2/98)
p<0.0001.
sPGA 0/1 at Week 16
UltIMMa-1:
SKYRIZI 88% (267/304), placebo 8% (8/102)
UltIMMa-2:
SKYRIZI 84% (246/294), placebo 5% (5/98)
NRI=Non-responder imputation.
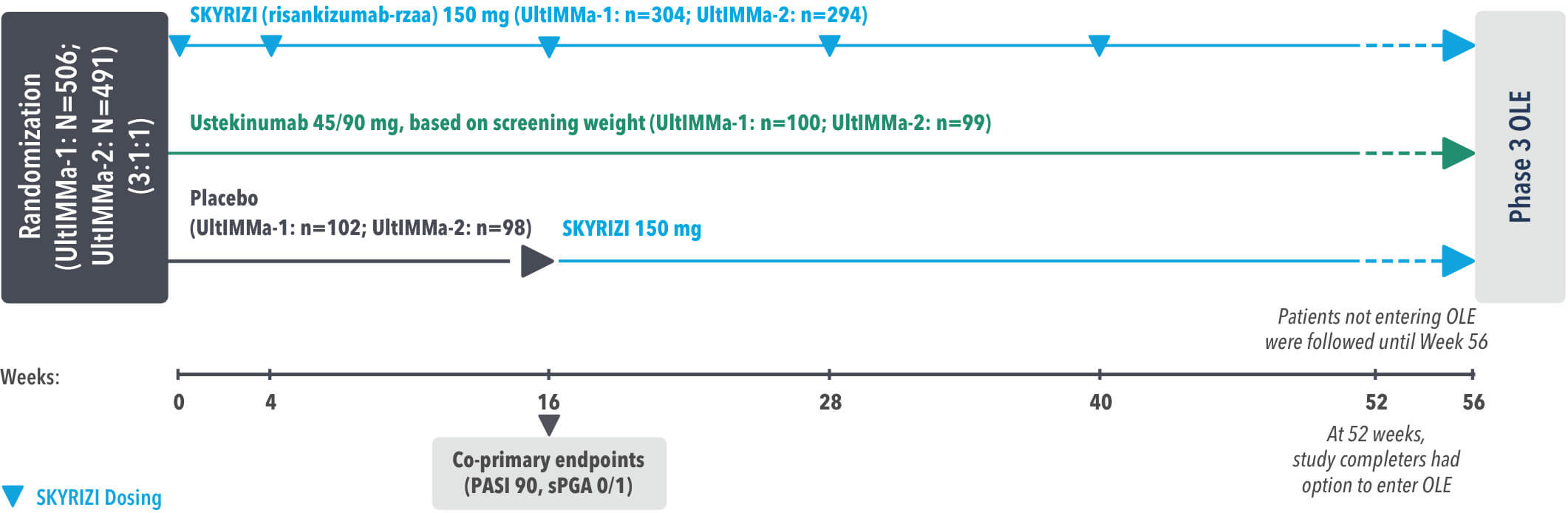
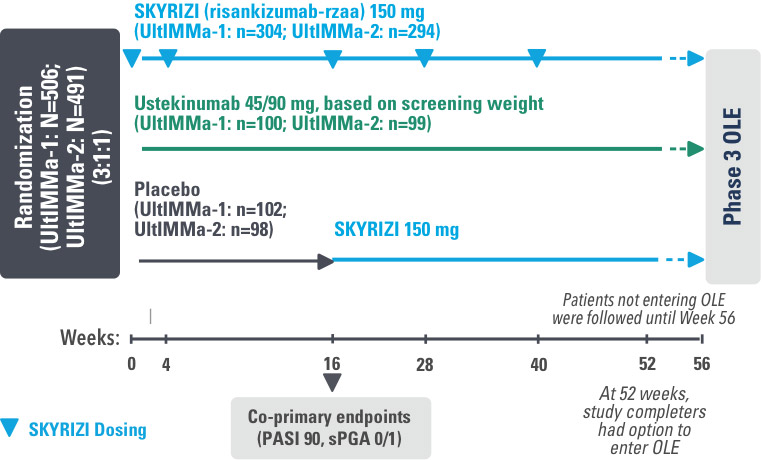
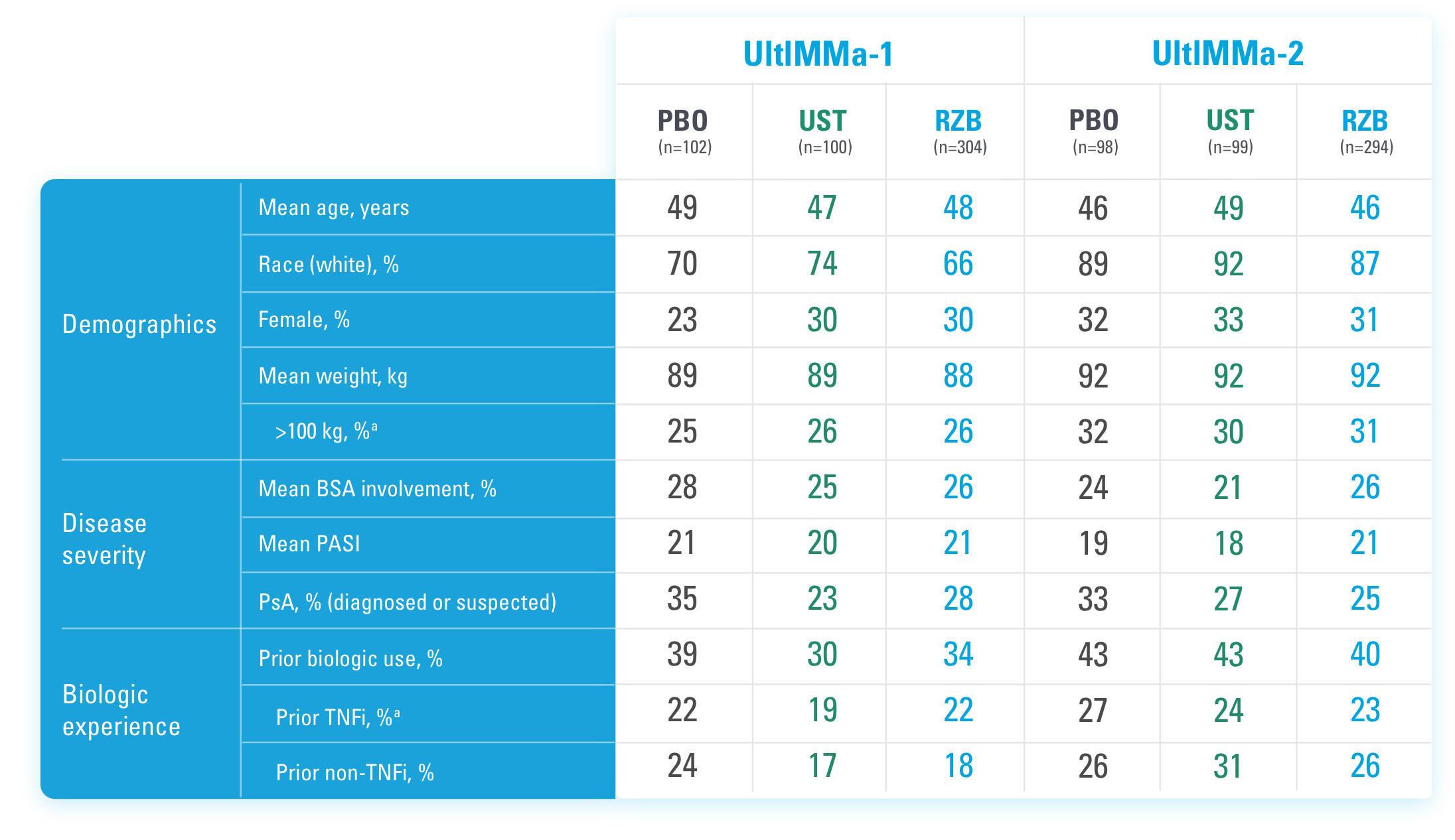
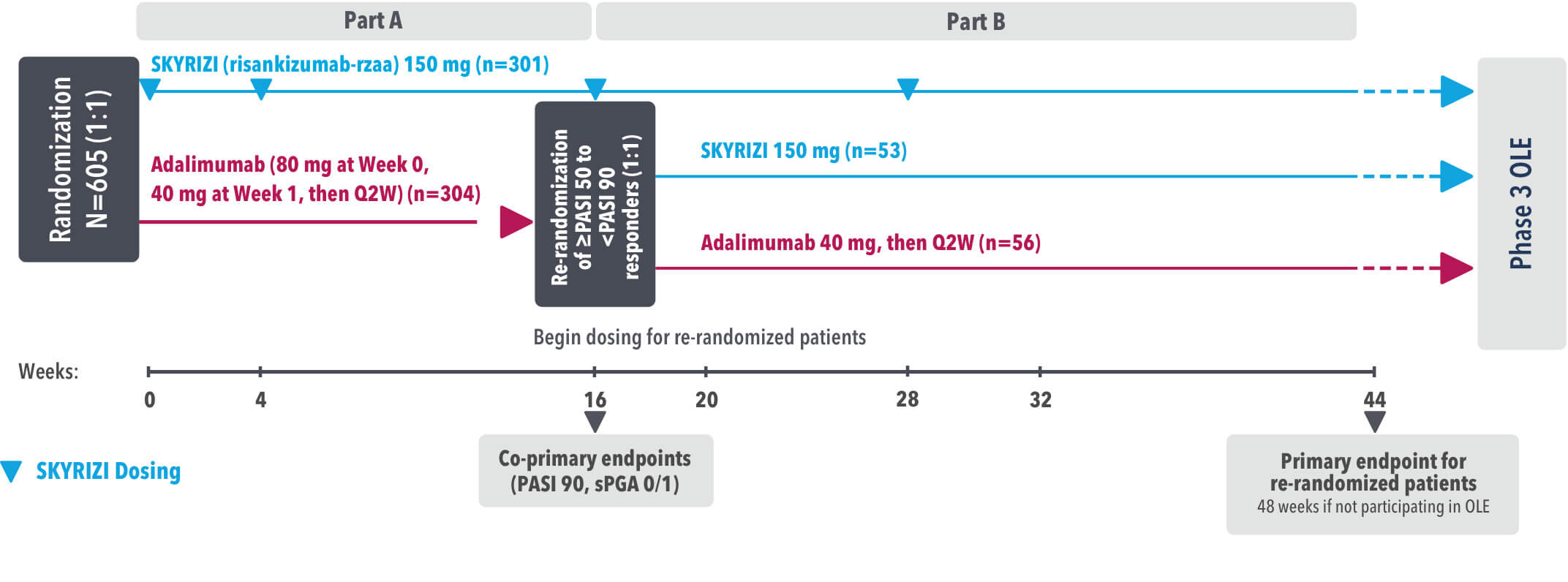
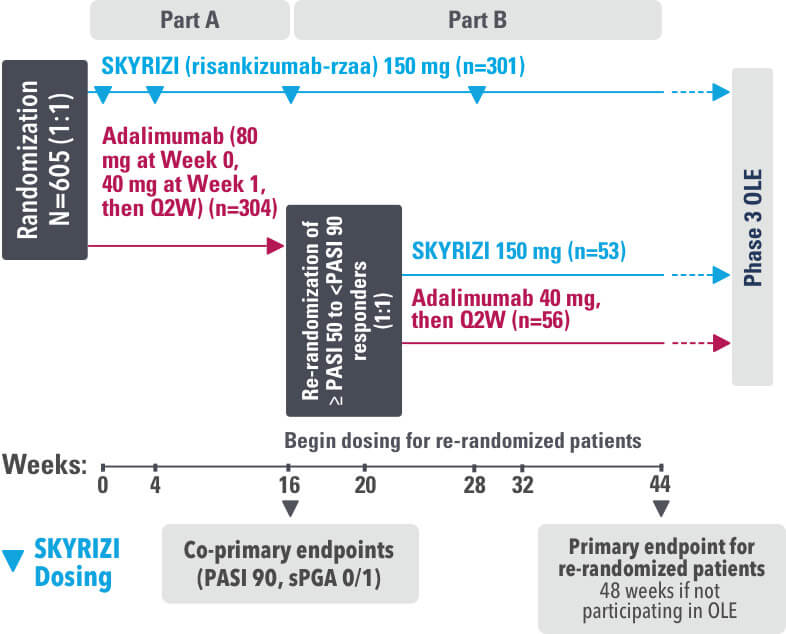
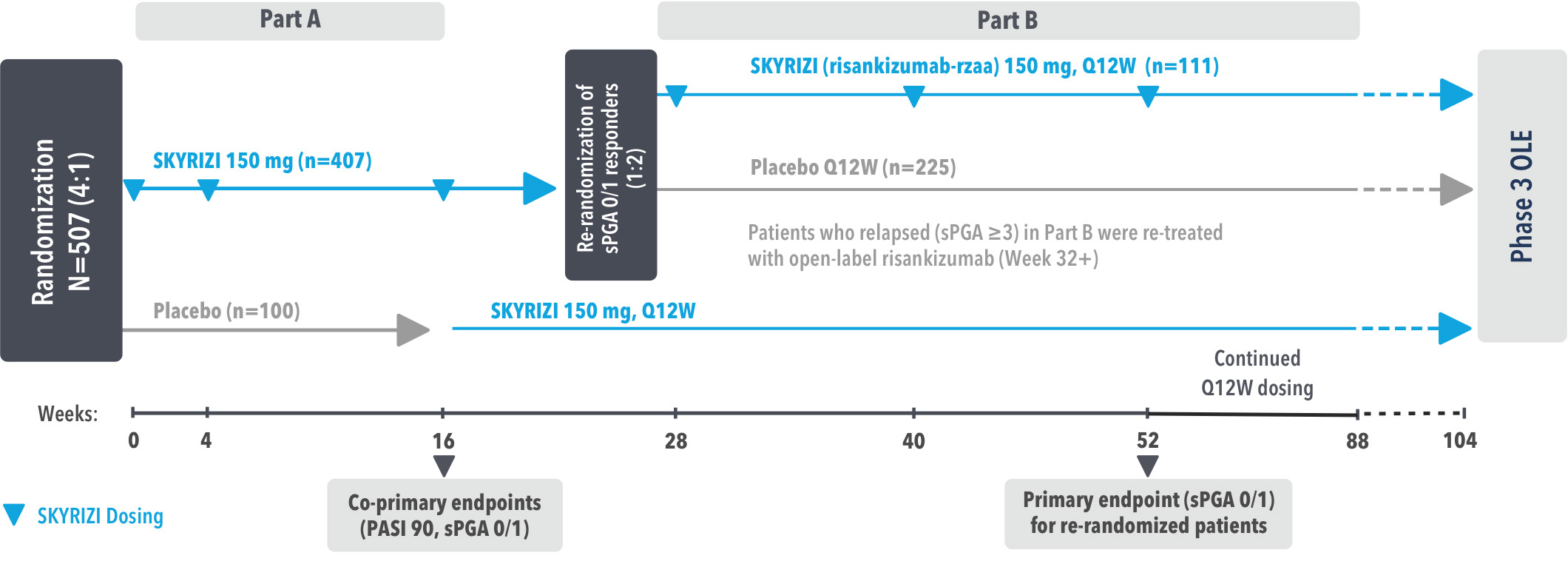
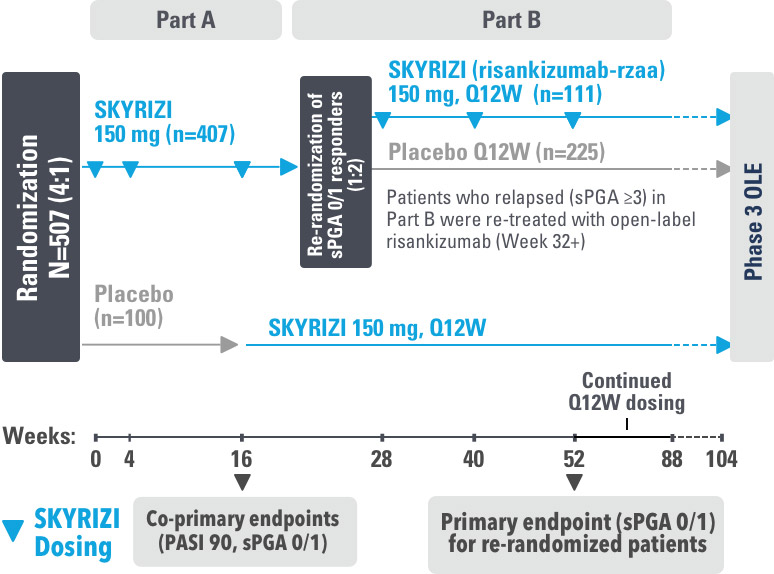
Study Design:
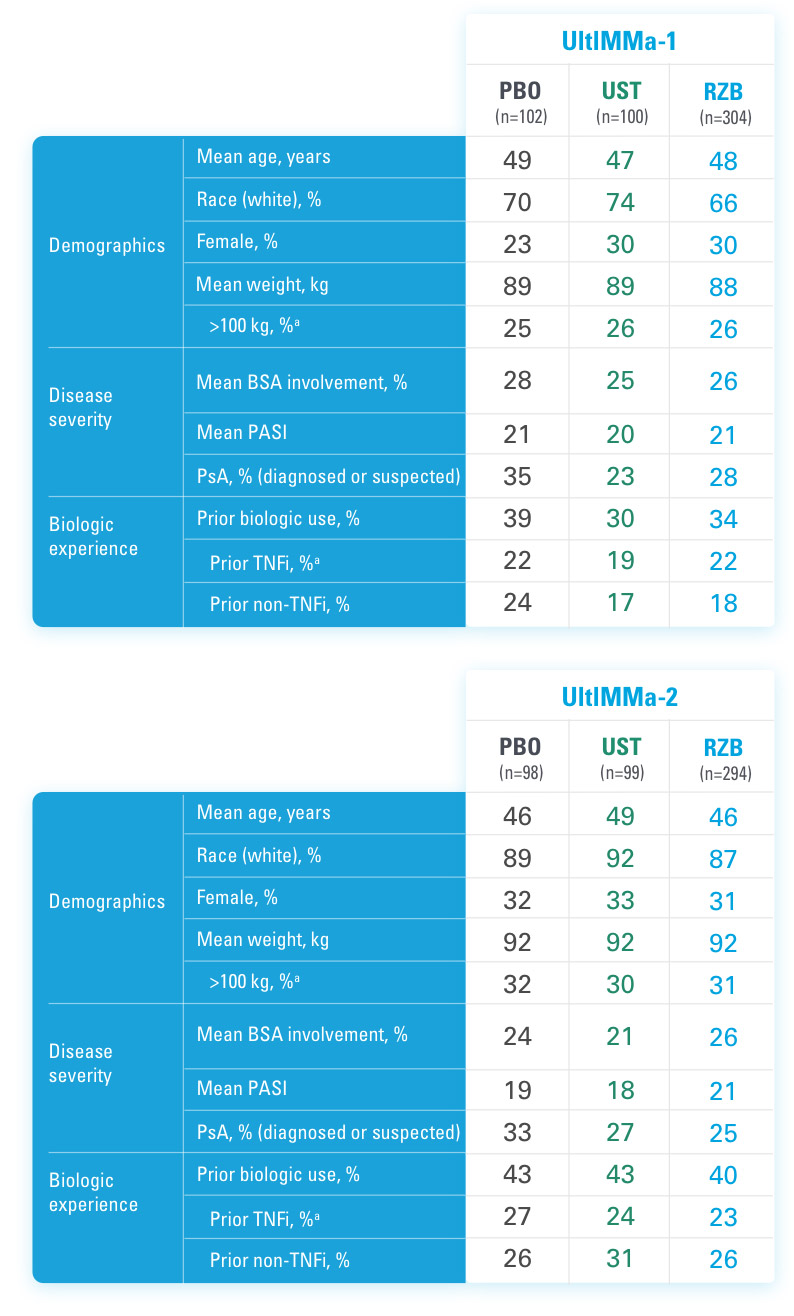
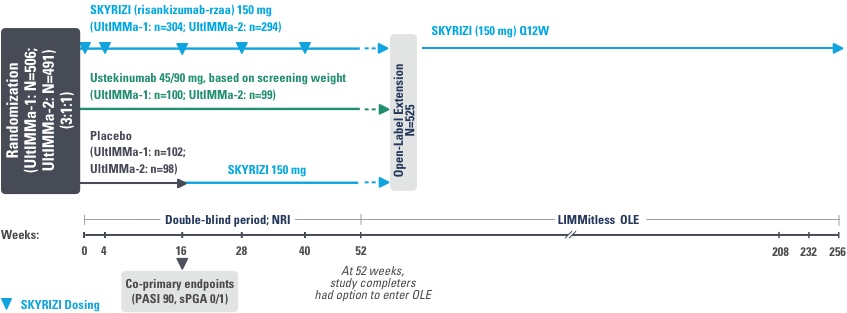
UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate Phase 3, randomized, double-blind, placebo- and active-controlled studies to evaluate the efficacy and safety of SKYRIZI (150 mg) vs placebo over 16 weeks and biologic active control (45 mg or 90 mg, based on screening weight) over 52 weeks in adult patients with moderate to severe plaque psoriasis. Patients received SKYRIZI 150 mg at Week 0, Week 4, and every 12 weeks thereafter.1,3
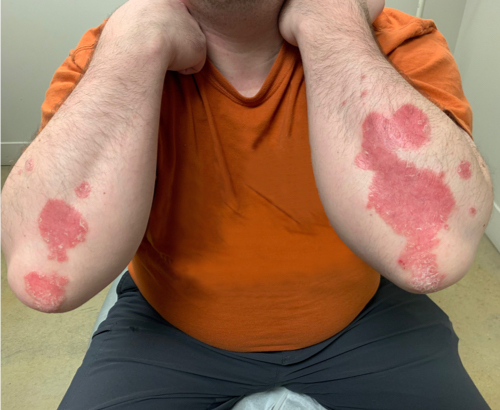
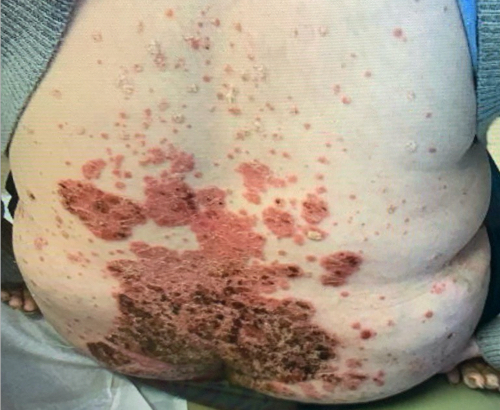
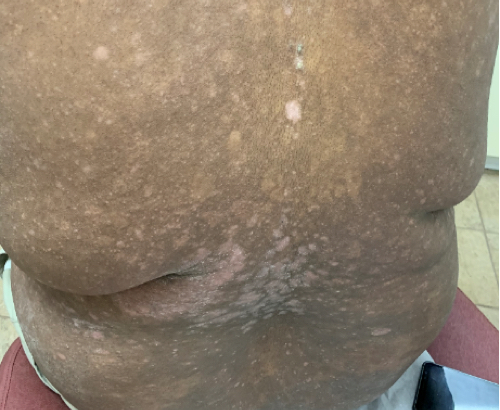
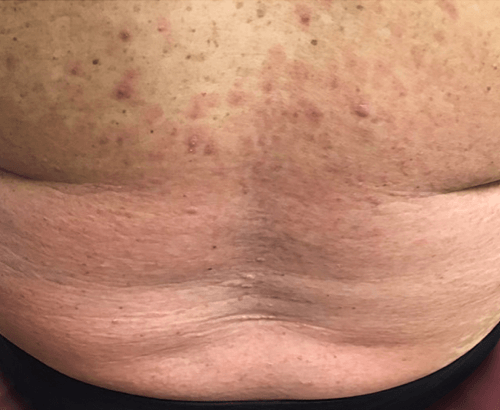
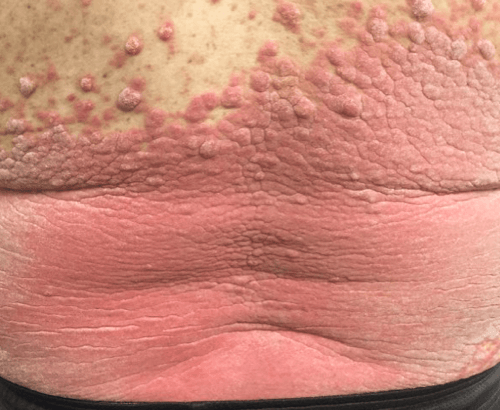
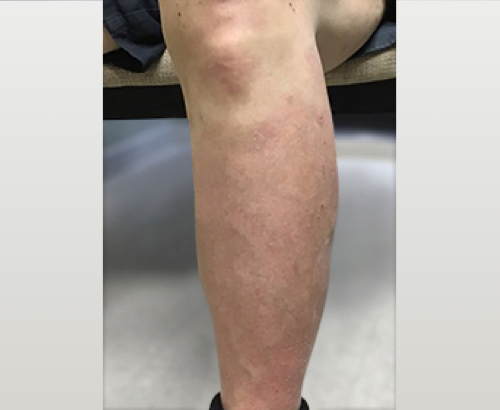
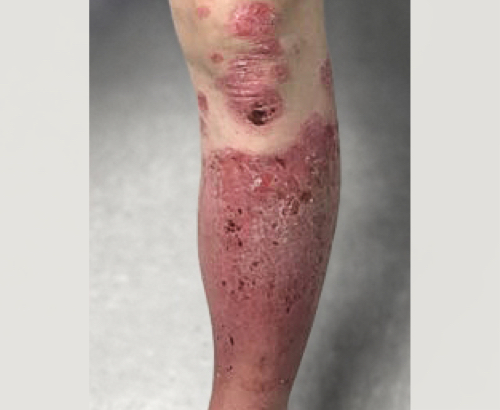
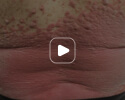
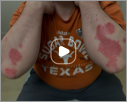
Week 16
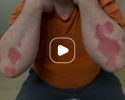
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. Meyer Horn.2
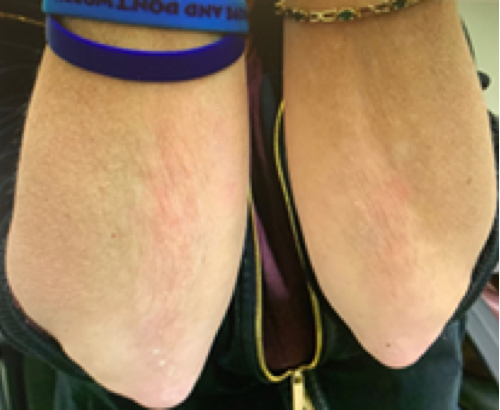
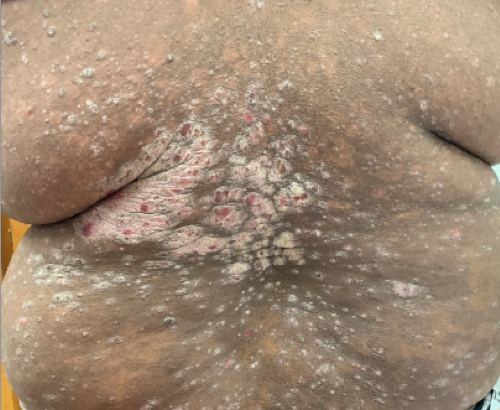
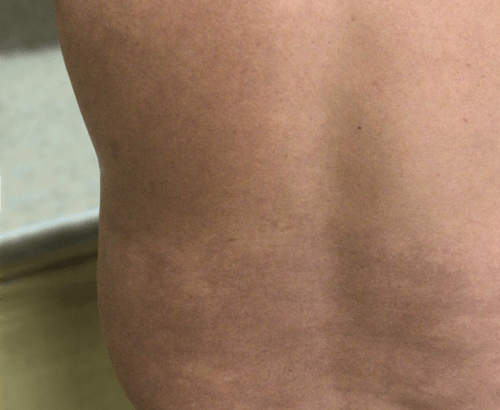
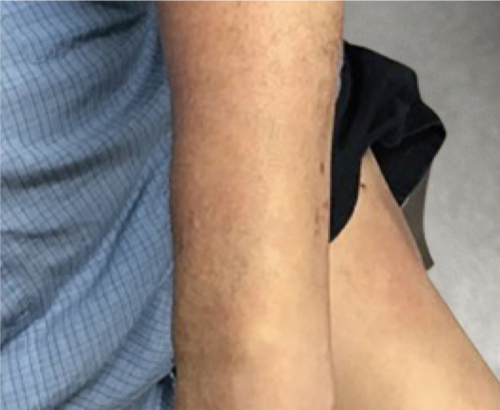
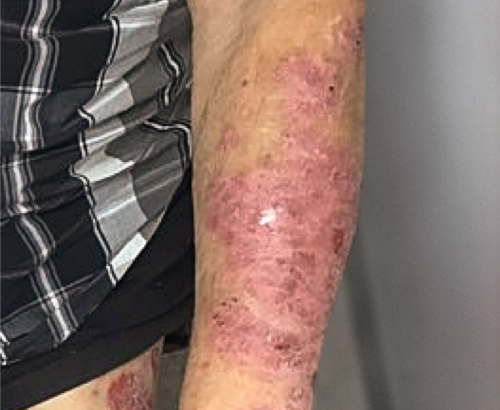
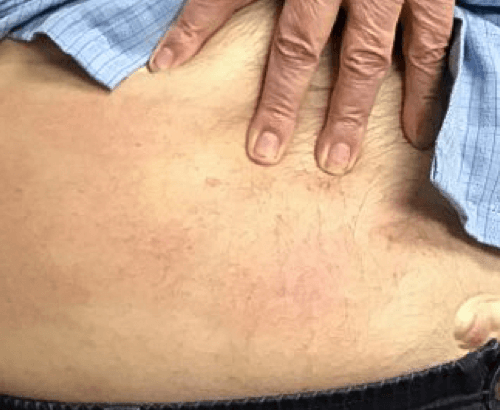
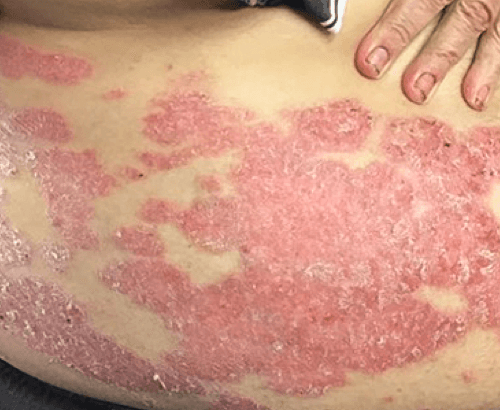
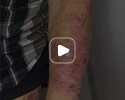
Week 16
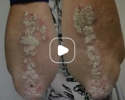
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Matthew Insley, RPA-C.2
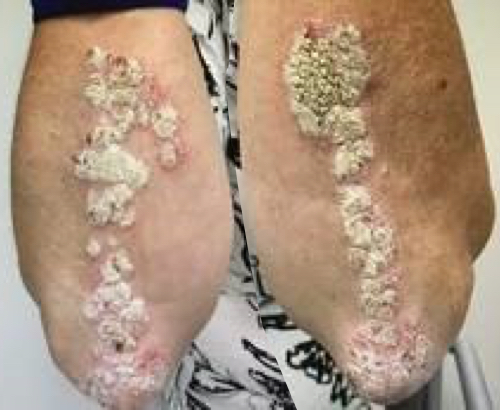
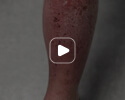
Week 16
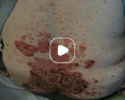
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Victor Czerkasij, FNP-C.2
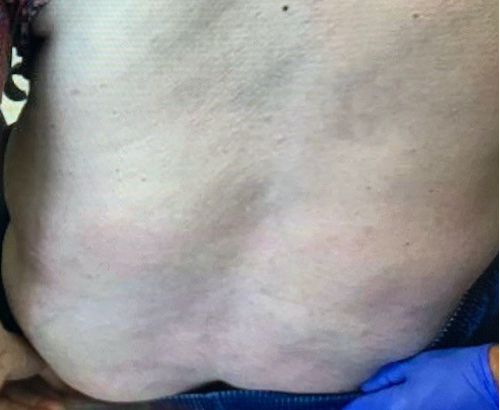
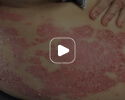
Week 16
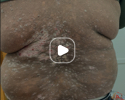
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. Ellen Frankel.2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. Matthew L. Miller2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. David Andrew Kasper2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Matthew Bruno, PA-C2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Matthew Bruno, PA-C2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Matthew Bruno, PA-C2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. Meyer Horn.2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Matthew Insley, RPA-C.2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Victor Czerkasij, FNP-C.2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. Ellen Frankel.2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. Matthew L. Miller2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. David Andrew Kasper2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Matthew Bruno, PA-C2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Matthew Bruno, PA-C2
Week 16
Patient results after 2 doses at Week 16
Patient photos representing results captured at baseline and Week 16, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Matthew Bruno, PA-C2
Week 52
Patient results after 5 doses at Week 52
Patient photos representing results captured at baseline and Week 52, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. Meyer Horn.2
Week 52
Patient results after 5 doses at Week 52
Patient photos representing results captured at baseline and Week 52, outside of clinical trials. PASI and sPGA achievement undefined.2
The patient depicted here has moderate to severe plaque psoriasis with an affected body surface area ≥10%.
Photos courtesy of Dr. Meyer Horn.2
4 doses per year
3-month dosing after 2 initiation doses at
Weeks 0 and 4 (150 mg/dose)1