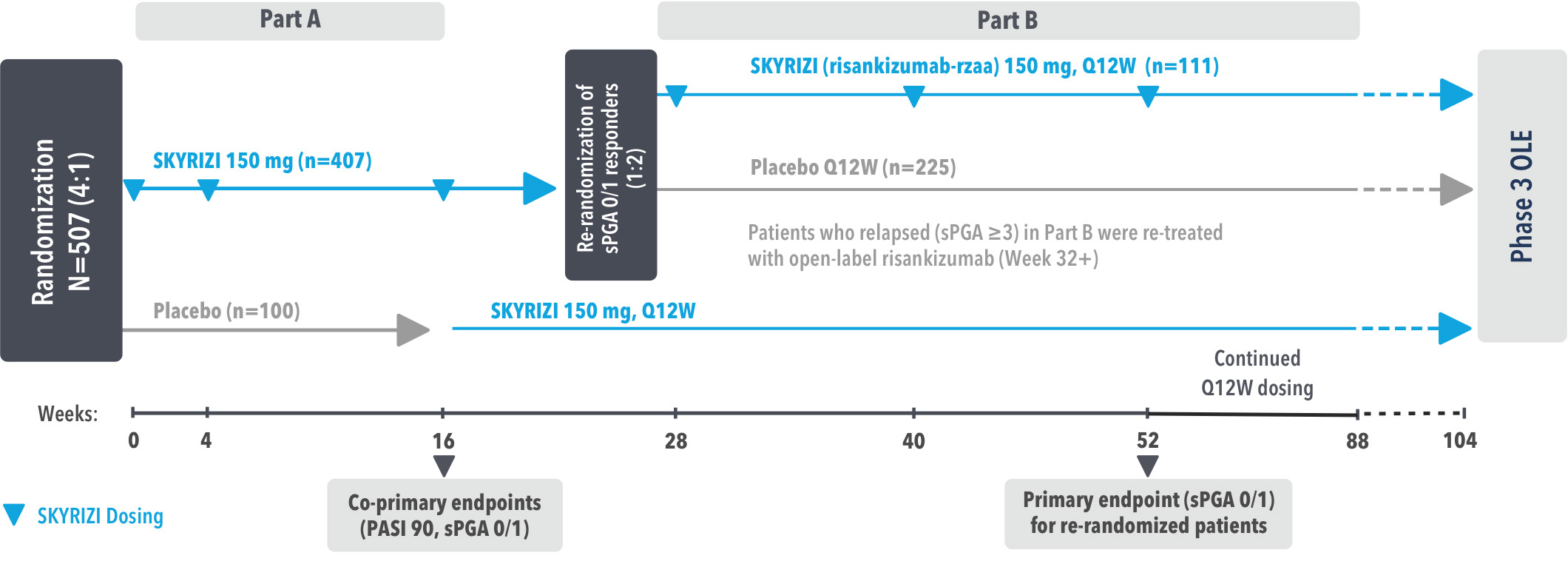
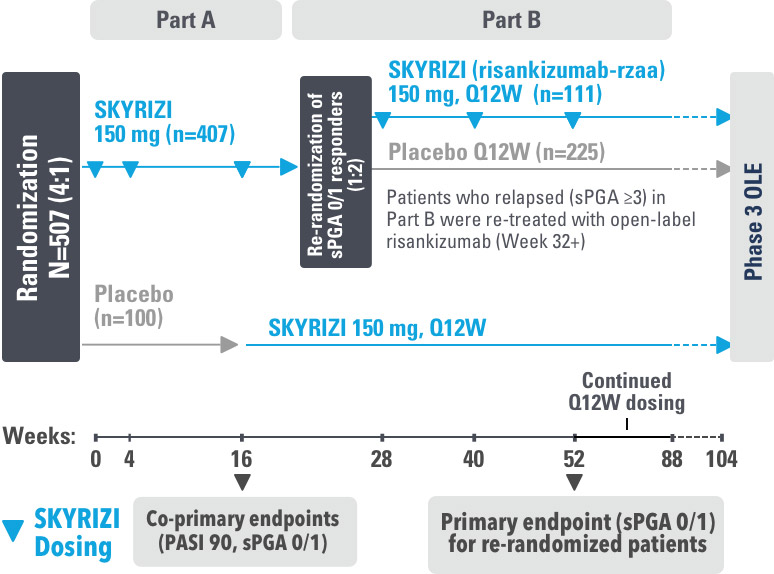
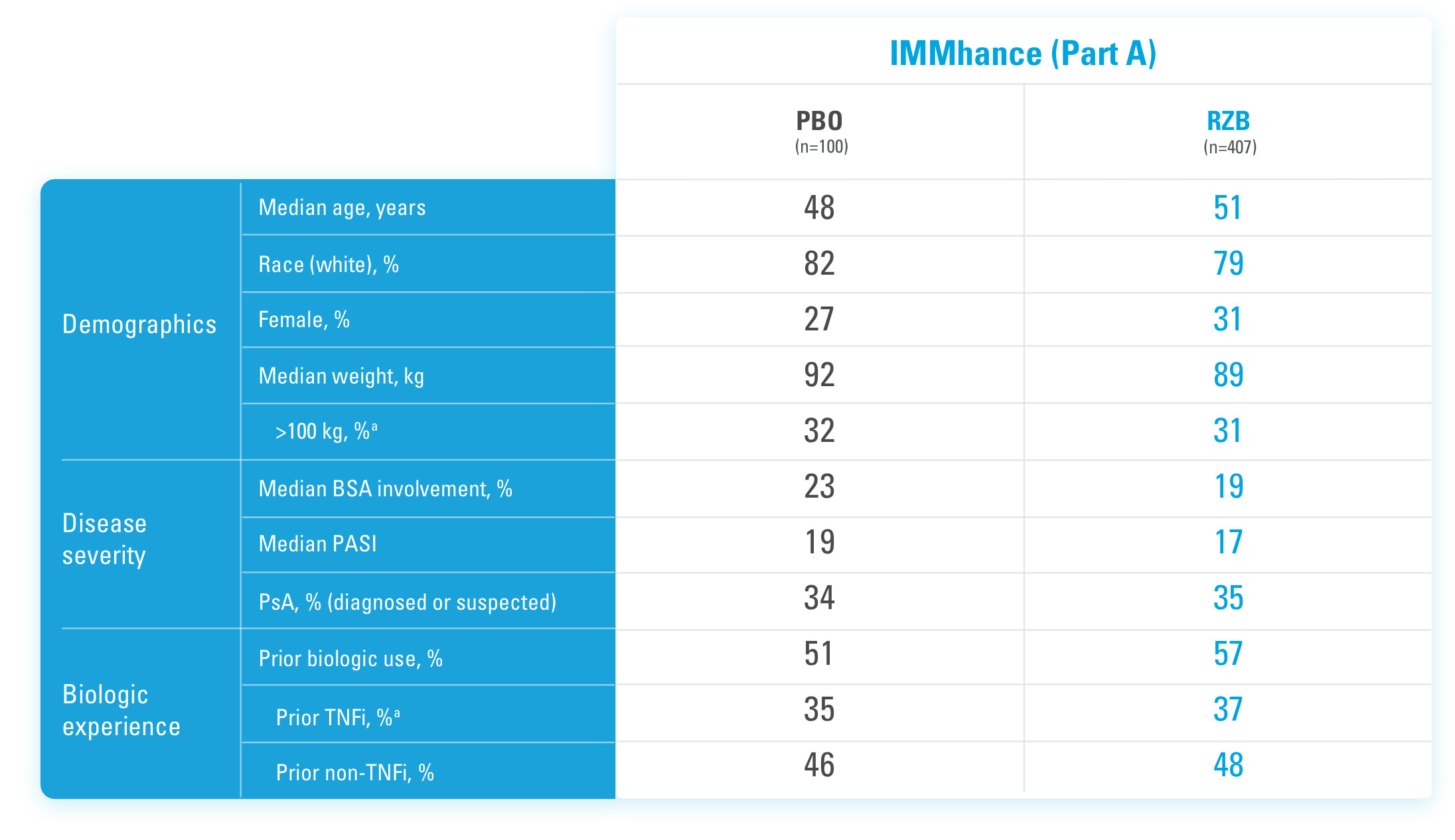
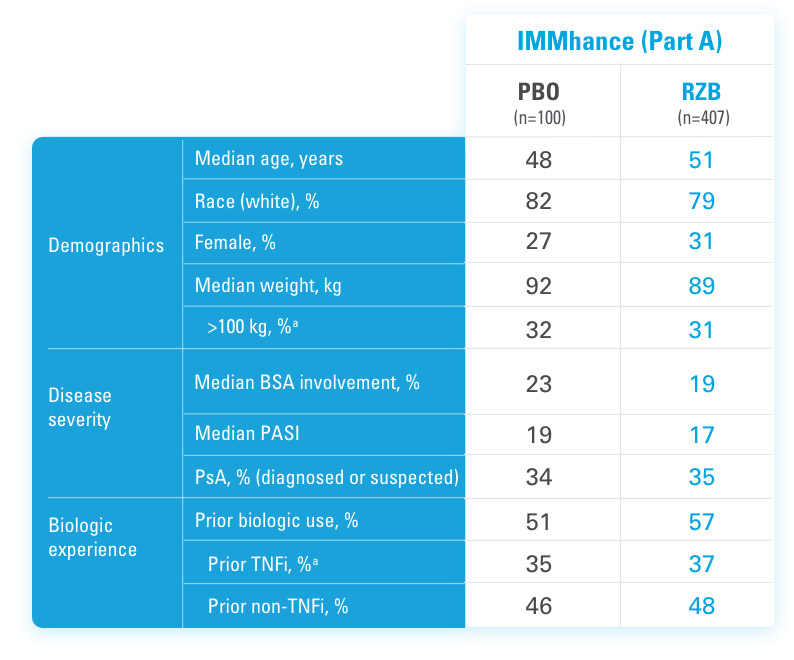
SKYRIZI COMPLETE ENROLLMENT

Resources for
Patient Access & support
YOUR SOURCE FOR SUPPORT
TO GET PATIENTS TIMELY ACCESS TO SKYRIZI

Ready to enroll your patient in Skyrizi Complete and start the process?

BILLING AND CODING
Clear guidance on billing and coding for SKYRIZI, including new NDC codes.

ACCESS AND REIMBURSEMENT
Forms and instructions to help patients with access and coverage.

SPECIALTY PHARMACIES
Contact details for specialty pharmacies

SPECIALTY PHARMACIES
Contact details for specialty pharmacies
This information is for informational purposes only and is not intended to provide reimbursement or legal advice. The information presented here does not guarantee payment or coverage.
SKYRIZI COMPLETE ENROLLMENT AND PRESCRIPTION FORM
- Download and fill out the Skyrizi Complete Enrollment and Prescription form with your patient
- After submitting the form via fax, your patient will receive a call from a Nurse Ambassador*
- You may also complete the Pharmacy Prescription Form and fax it to your patient's specialty pharmacy
GUIDE TO STARTING SKYRIZI
Reference this guide for a comprehensive list of steps to take and communication to expect when starting a patient on SKYRIZI.
PRIOR AUTHORIZATION INSTRUCTIONS
Follow this helpful checklist to request coverage for SKYRIZI.
*Nurse Ambassadors are provided by AbbVie and do not provide medical advice or work under the direction of the prescribing health care professional (HCP). They are trained to direct patients to speak with their HCP about any treatment-related questions, including further referrals.
GUIDE TO BILLING AND CODING
Your guide to relevant codes (including commercial and Medicare) as well as helpful tips for completing forms.
NDC CODES
| SKYRIZI Pen 150 mg/mL |
|---|
| 0074-2100-01 Carton of 1 |
| SKYRIZI Prefilled Syringe 150 mg/mL |
|---|
| 0074-1050-01 Carton of 1 |
ICD-10-CM DIAGNOSIS CODE2†‡
Plaque Psoriasis
| ICD-10-CM code | Description |
|---|---|
| L40.0 | Psoriasis vulgaris |
| L40.8 | Flexural psoriasis |
| L40.9 | Psoriasis, unspecified |
Psoriatic Arthritis
| ICD-10-CM code | Description |
|---|---|
| L40.5 | Arthropathic psoriasis |
| L40.50 | Arthropathic psoriasis, unspecified |
| L40.51 | Distal interphalangeal psoriatic arthropathy |
| L40.52 | Psoriatic arthritis mutilans |
| L40.59 | Other psoriatic arthropathy |
HEALTHCARE COMMON PROCEDURE CODING SYSTEM (HCPCS) CODES3
| HCPCS code | Description | Payer type |
|---|---|---|
| J3590 | Unclassified biologics | Commercial, Medicare |
| C9399 | Unclassified drugs or biologics | Medicare |
ICD-10-CM=International Classification of Diseases, Tenth Revision, Clinical Modification.
†The codes shown above are only general suggestions and are not intended to encourage or suggest a use of any drug that is inconsistent with FDA-approved use.
‡This information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Providers are encouraged to contact third-party payers for specific information about their coverage policies.
Along with support from Skyrizi Complete, you can use the forms here to help patients with access and coverage for SKYRIZI.
APPEALS LETTER SAMPLE
Appeal a denied claim for SKYRIZI.
TEMPLATE
FORMULARY EXCEPTION LETTER
Request a formulary exception to allow coverage for SKYRIZI.
TEMPLATE
HIPAA AUTHORIZATION
Allow patients to authorize the release of health information related to their treatment with SKYRIZI.
TEMPLATE
CONTACT DETAILS FOR SPECIALTY PHARMACIES
A complete list of specialty pharmacies that provide product-specific support for SKYRIZI.
Use these guides & best practices to help
get patients timely access to SKYRIZI

SKYRIZI ONBOARDING

ADDITIONAL RESOURCES

INJECTION SUPPORT VIDEOS

Injection Training Quick Tips
The resources on this page are provided for informational purposes only and are not intended as reimbursement or legal advice. The information presented here does not guarantee payment or coverage.
SKYRIZI COMPLETE ENROLLMENT AND PRESCRIPTION FORM
Download and fill out the Skyrizi Complete Enrollment and Prescription Form with your patient. After submitting the form via fax, your patient will receive a call from a Nurse Ambassador.* You may also complete the Pharmacy Prescription Form and fax it to your patient's specialty pharmacy.
*Nurse Ambassadors are provided by AbbVie and do not provide medical advice or work under the direction of the prescribing health care professional (HCP). They are trained to direct patients to speak with their HCP about any treatment-related questions, including further referrals.
BENEFITS VERIFICATION CHART
Help patients confirm their insurance coverage and out-of-pocket costs.
INSURANCE COMPARISON
Simple steps to help patients choose their insurance coverage when it's time to pick a plan.
PATIENT BROCHURE
Prepare patients for treatment with SKYRIZI with information on plaque psoriasis, SKYRIZI efficacy, and more.
SKYRIZI COMPLETE BROCHURE
Information about the resources available from Skyrizi Complete, including Nurse Ambassadors, injection support, and possible ways to save.*
AAD RECOMMENDATIONS FOR TELEMEDICINE PREPAREDNESS
Tips for meeting with a dermatologist via phone or video chat from the American Academy of Dermatology Association.
PASI SCORE CALCULATOR
Allows patients to enter the locations and severity of their symptoms and produce their current PASI score.
*Nurse Ambassadors are provided by AbbVie and do not provide medical advice or work under the direction of the prescribing health care professional (HCP). They are trained to direct patients to speak with their HCP about any treatment-related questions, including further referrals.
A quick-tip guide for patients on how to inject, whether they have injected before or are new to it.

150 mg/mL SKYRIZI PEN

150 mg/mL PREFILLED SYRINGE
looking for more resources?Offer enrollment to patients and submit forms
electronically with CompletePro.com
By registering through CompletePro.com, you can choose the
capabilities that are most relevant to you and your patients’ needs, such as:
Instant benefits verification:
- Patient out-of-pocket costs
- Any prior authorization requirements
- Pharmacy options available to the patient
- Patient eligibility for any drug discount from the pharmaceutical company
Online prescribing efficiencies:
- Complete a Prior Authorization and send it directly to the insurer
- Send a prescription directly to the patient's chosen pharmacy
- Send Skyrizi Complete Savings Card to your patient's preferred specialty pharmacy (with or without a prescription)
- Be notified via text, email, or website in advance of patient's prior authorization expiration
- Easily access each patient's prescription fill status
To get started with Complete Pro, speak with your Sales Representative or call Technical Support at 1-877-COMPLETE (1-877-266-7538)