More skyrizi patients saw endoscopic REMISSION vs placebo1,4
Endoscopic Remission: SES-CD ≤4 and at least a 2-point reduction versus baseline and no subscore greater than 1 in any individual variable, as scored by a central reviewer.

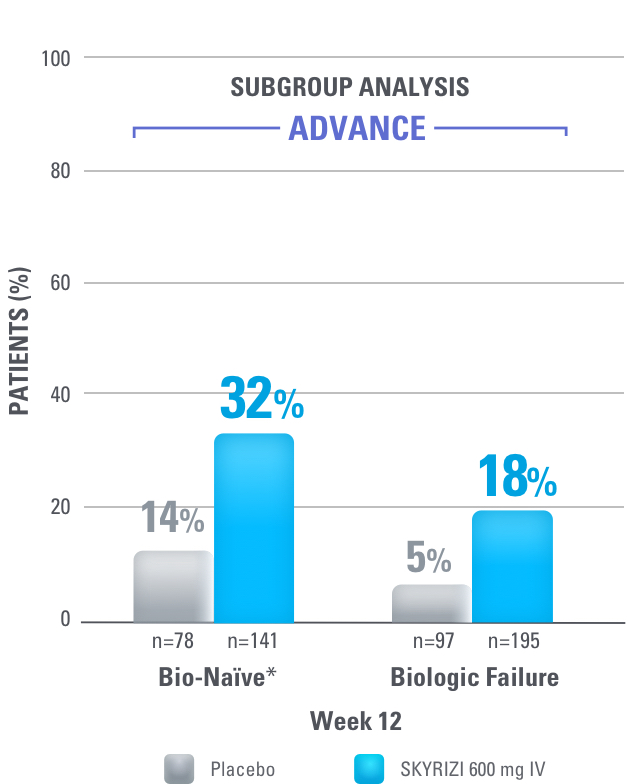
24% OF TOTAL PATIENTS DEMONSTRATED ENDOSCOPIC REMISSION WITH SKYRIZI AT WEEK 121
Ranked Secondary Endpoint for ADVANCE:
Endoscopic Remission AT WEEK 12
(Total Population):
24% SKYRIZI vs 9% placebo, p<0.001
Endoscopic Remission: SES-CD ≤4 and at least a 2-point reduction versus baseline and no subscore greater than 1 in any individual variable, as scored by a central reviewer.
ENDOSCOPIC REMISSION DATA: POST HOC DATA OF SUBGROUP ANALYSIS1,5
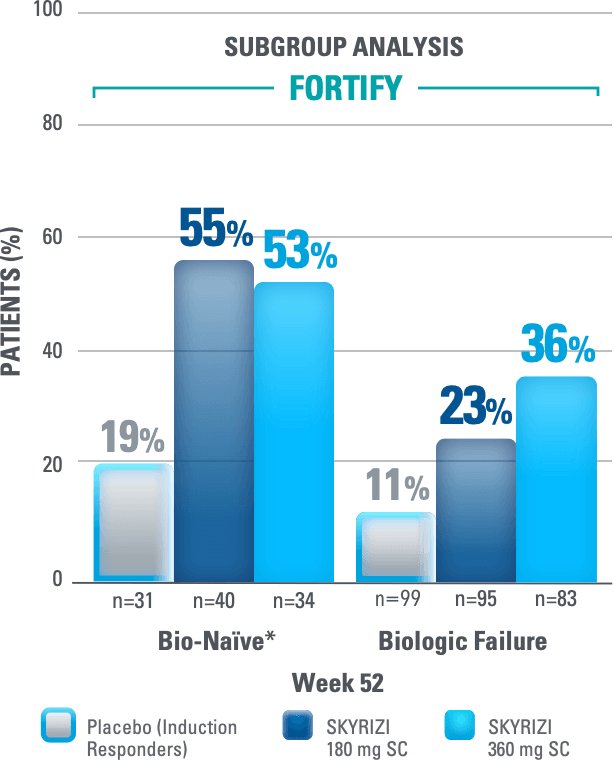
Data Limitations: Endoscopic remission sub-group data at Week 52 was not tested for multiplicity control, and cannot demonstrate a statistically significant difference in treatment effect for SKYRIZI vs placebo (induction responders). No statistical or clinical conclusions can be made.
Endoscopic Remission: SES-CD ≤4 and at least a 2-point reduction versus baseline and no subscore greater than 1 in any individual variable, as scored by a central reviewer.
Endoscopic remission was observed at Week 52 in 41% (48/117) of subjects treated with the SKYRIZI 360 mg maintenance regimen and 13% (17/130) of subjects treated with placebo. This endpoint was not statistically significant under the pre-specified multiple testing procedure.
Endoscopic remission was observed at Week 52
Secondary Endpoints for FORTIFY:
Endoscopic Remission AT WEEK 52:
(Total Population):
33% SKYRIZI 180 mg SC vs
13% placebo (induction responders)
41% SKYRIZI 360 mg SC vs
13% placebo (induction responders)
This endpoint was not statistically significant under the prespecified multiple testing procedure.
Placebo (Induction Responders): Patients who achieved CDAI clinical response (CR-100)† to SKYRIZI induction therapy and were randomized to receive placebo in the maintenance study
Data Limitations: Endoscopic remission and sub-group data at Week 52 was not tested for multiplicity control, and cannot demonstrate a statistically significant difference in treatment effect for SKYRIZI vs placebo (induction responders). No statistical or clinical conclusions can be made.
Endoscopic Remission: SES-CD ≤4 and at least a 2-point reduction versus baseline and no subscore greater than 1 in any individual variable, as scored by a central reviewer.